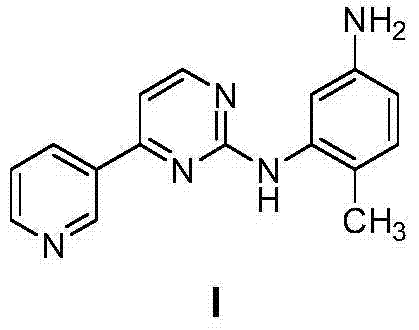
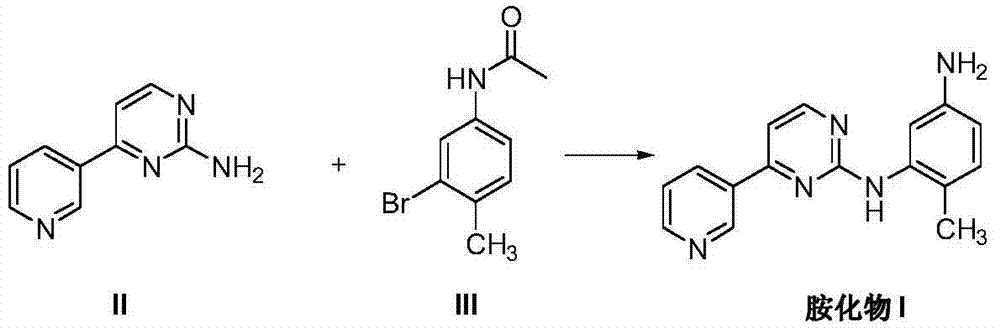
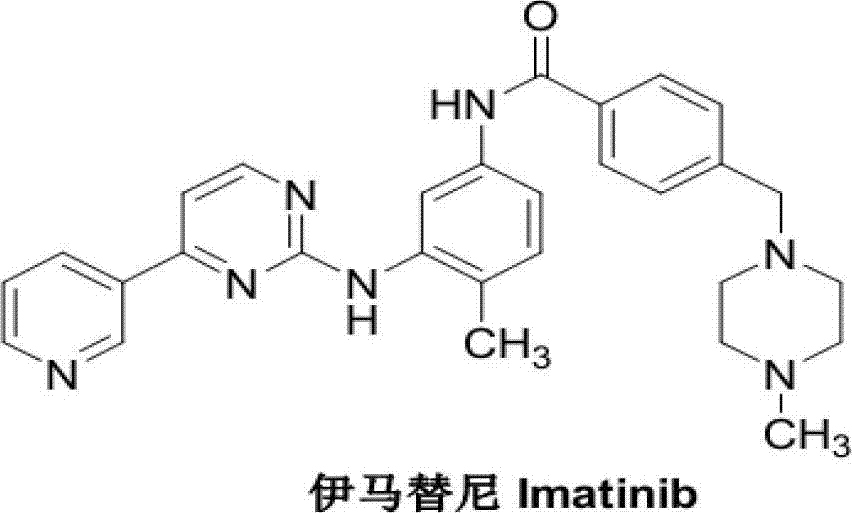
Method for preparing imatinib mesylate intermediate
A technology for imatinib mesylate and intermediates, which is applied in the field of preparation of imatinib mesylate intermediates, can solve the problems of reduced yield and many steps, and achieve reduced manufacturing steps, reduced costs, and improved Effects of Atom Economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]Under nitrogen protection, add 400 mL of dioxane as a solvent into a 1L three-necked flask, start stirring, and then add 4-(3-pyridyl)pyrimidin-2-amine (20.7 g, 0.12 mol) and CuI (5.7 g, 0.03mol), anhydrous potassium carbonate (20.7g, 0.3mol). After stirring at room temperature for 15 minutes, 2-bromo-4-acetamido-toluene (22.7 g, 0.10 mol) of formula III and DMEDA (4.0 mL, 0.04 mol) were further added. The temperature was raised to reflux, and the reflux was maintained for 20 hours. After cooling to room temperature, trifluoroacetic acid (9.2 mL, 0.12 mol) was added to the system, stirred at room temperature for 2 hours, and the reaction was completed. Adjust the pH to pH=8-9 with concentrated ammonia water. Add 400mL of ethyl acetate saturated with 100mL of saturated saline, stir, stand still and separate layers, the aqueous phase is washed three times with ethyl acetate (100mLx3), the organic phases are combined, dried over anhydrous magnesium sulfate, concentrated t...
Embodiment 2
[0047] Under nitrogen protection, add 400 mL of dioxane as a solvent into a 1L three-necked flask, start stirring, and then add 4-(3-pyridyl)pyrimidin-2-amine (20.7 g, 0.12 mol) and CuI (5.7 g, 0.03mol), anhydrous potassium carbonate (20.7g, 0.3mol). After stirring at room temperature for 15 minutes, 2-bromo-4-acetamido-toluene III of formula III (22.7 g, 0.10 mol) and DMEDA (4.0 mL, 0.04 mol) were further added. The temperature was raised to reflux, and the reflux was maintained for 20 hours. After cooling to room temperature, 6N HCl (20 mL) was added to the system, heated to 50° C., stirred for 1 hour, and the reaction was completed. Adjust the acidity and alkalinity to pH=8-9 with concentrated ammonia water. Add 400 mL of ethyl acetate and 100 mL of saturated brine, stir, stand still and separate layers, wash the aqueous phase with ethyl acetate three times (100 mL x 3), combine the organic phases, dry over anhydrous magnesium sulfate, concentrate to give a yellow solid, ...
Embodiment 3
[0049] Under nitrogen protection, add 400 mL of dioxane as a solvent into a 1L three-necked flask, start stirring, and then add 4-(3-pyridyl)pyrimidin-2-amine (20.7 g, 0.12 mol) and CuI (5.7 g, 0.03mol), anhydrous potassium carbonate (20.7g, 0.3mol). After stirring at room temperature for 15 minutes, 2-bromo-4-acetamido-toluene (22.7 g, 0.1 mol) of formula III and DMEDA (4.0 mL, 0.04 mol) were further added. The temperature was raised to reflux, and the reflux was maintained for 20 hours. Cool to 0°C, add 10mL of 30% sodium hydroxide solution to the system, keep at 0°C, stir and react for 1 hour, and the reaction ends. First adjust the pH to pH=8-9 with dilute hydrochloric acid. Add 400mL of ethyl acetate saturated with 100mL of saturated saline, stir, stand still and separate layers, the aqueous phase is washed three times with ethyl acetate (100mLx3), the organic phases are combined, dried over anhydrous magnesium sulfate, concentrated to give a brownish yellow solid, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com