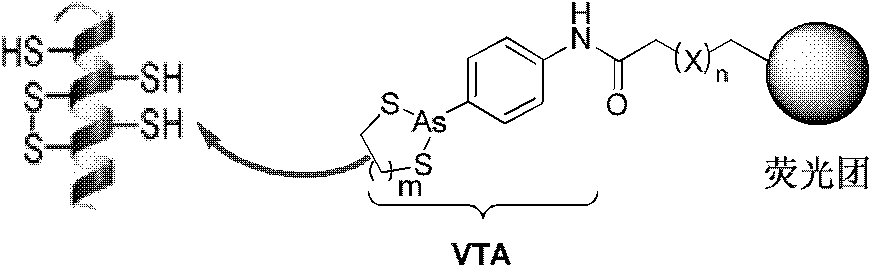
Compound and method for in-situ detecting o-sulfhydryl protein in organism
A compound and protein technology, which is applied in the field of in situ detection of adjacent thiol proteins in organisms, can solve the problems of not being able to detect adjacent thiol proteins in situ, and achieve good redox tolerance and good fat solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
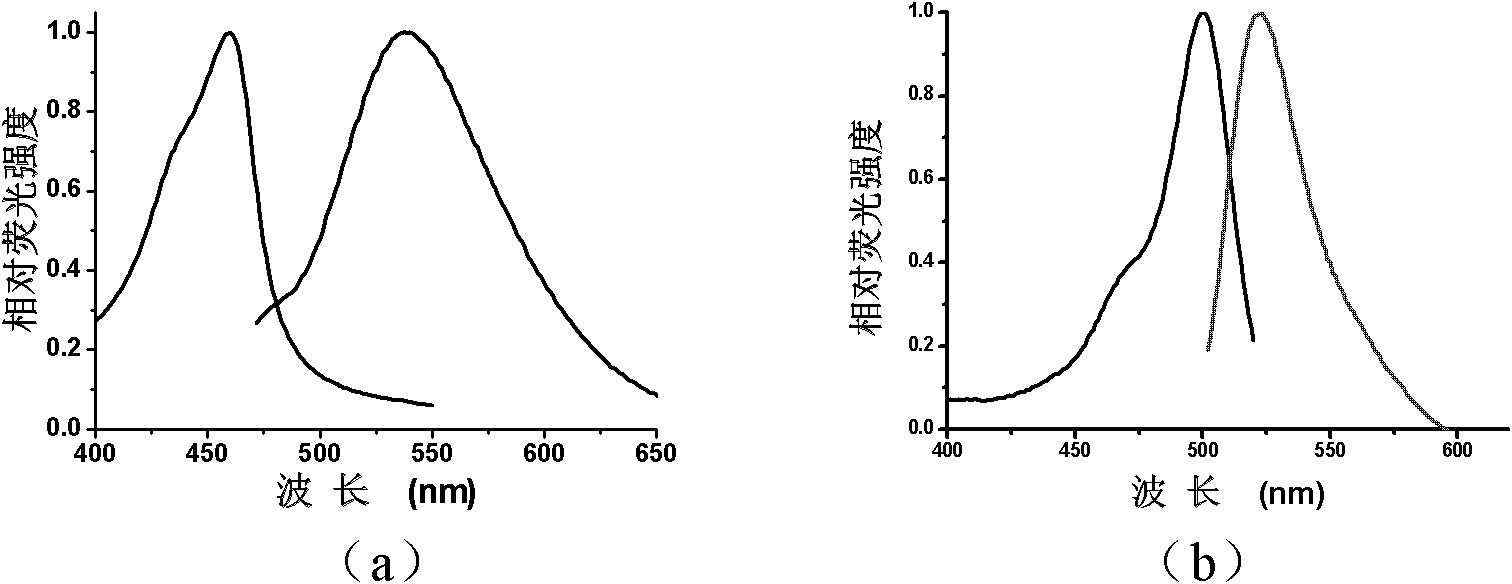
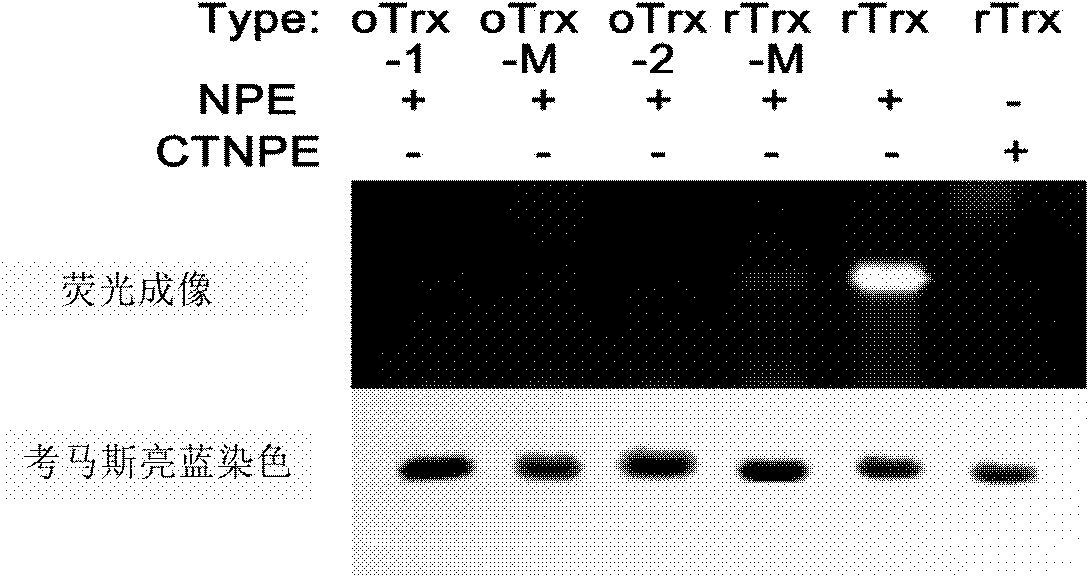
Image
Examples
Embodiment 1
[0072] The synthetic route of the target probe using naphthalimide as fluorophore is as follows:
[0073]
[0074] VTA1: p-aminophenylarsine (10.85g, 50mmol) was dissolved in methanol, heated to reflux to make the solution clear, and then phenylhydrazine (10.30mL, 100mmol) was added dropwise, and the addition was completed in 10 minutes. A large amount of nitrogen gas was generated, and when the nitrogen gas became low, the reflux was continued for 1 hour. The reaction solution was concentrated at 90°C, 85mL of water and 0.1M aqueous sodium hydroxide solution (85mL) were added, washed with diethyl ether (2×75mL), and aqueous ammonium chloride solution (1M, 40mL) was added to the aqueous phase, and placed in a refrigerator at 0°C to crystallize a large amount of White needle-like crystals of p-aminophenylarsine oxide were vacuum-dried to obtain 5.54 g of the product (yield 53%). Stored in KOH filled with Ar gas.
[0075] VTA2: VTA1 (1.24g, 6.43mmol) was dissolved in anhydr...
Embodiment 2
[0082] The synthesis route of the target probe using NBD as the fluorophore is as follows:
[0083]
[0084] S6: VTA4 (122 mg, 0.328 mmol) was dissolved in 10 mL of dichloromethane, triethylamine (75 μL) was added, after stirring, NBD-Cl (78.3 mg, 0.393 mmol) was dissolved in 8 mL of dichloromethane and slowly added dropwise into the S5 solution and stirred overnight at room temperature. The reaction solution turned from light yellow to dark brown. Spin-dry solvent column chromatography, dichloromethane / methanol = 20 / 1 to 10 / 1 gradient elution to obtain 58 mg of tan solid (yield 33%). 1 H NMR (DMSO-d 6 , 400MHz) δ1.40(q, 2H), 1.66(m, 4H), 2.31(t, 2H, J=7.2Hz), 3.47(t, 2H, J=6.0Hz,), 6.42(d, 1H, J=9.2Hz), 7.00(t, 1H, J=7.2Hz), 7.26(t, 2H, J=7.6Hz), 7.55(d, 2H, J=7.6Hz), 8.49(d, 1H, J= 8.8Hz).
Embodiment 3
[0086] The target probe synthesis using fluorescein as the fluorophore is as follows:
[0087]
[0088] S8: Fluorescein (3.32g, 10mmol), N-hydroxysuccinimide (1.41g, 12mmol) and DCC (3.15g, 15mmol) were dissolved in 10mL of dry DMF, heated to 70 ~80°C, react for 1 hour. Cooled in an ice bath, a large amount of crystals precipitated, filtered, and the by-product dicyclohexyl urea (white crystals) was filtered off, the filter cake was washed with acetone until white, discarded; the filtrate was a deep red solution, concentrated, and directly went to Next do the next reaction.
[0089] S9: S8 (2.49 g, 5.77 mmol) was dissolved in 10 mL of DMF, piperazine (0.99 g, 11.55 mmol) and triethylamine (2.4 mL, 17.32 mmol) were added. The reaction solution container was covered with aluminum foil to avoid light, and stirred at room temperature for 14 hours under the protection of argon. Neutralize with acetic acid. The solvent was spin-dried to obtain a dark red oil; column chromatog...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com