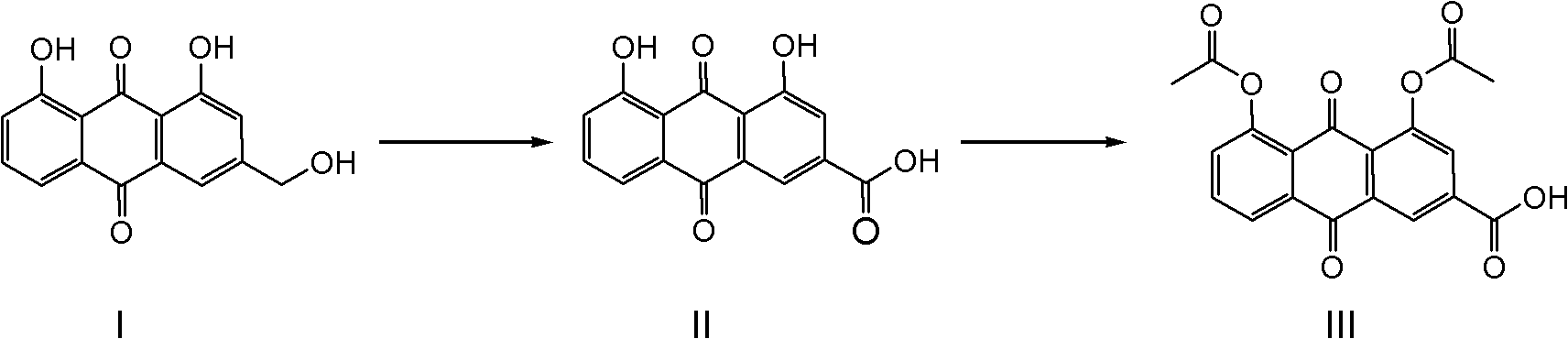
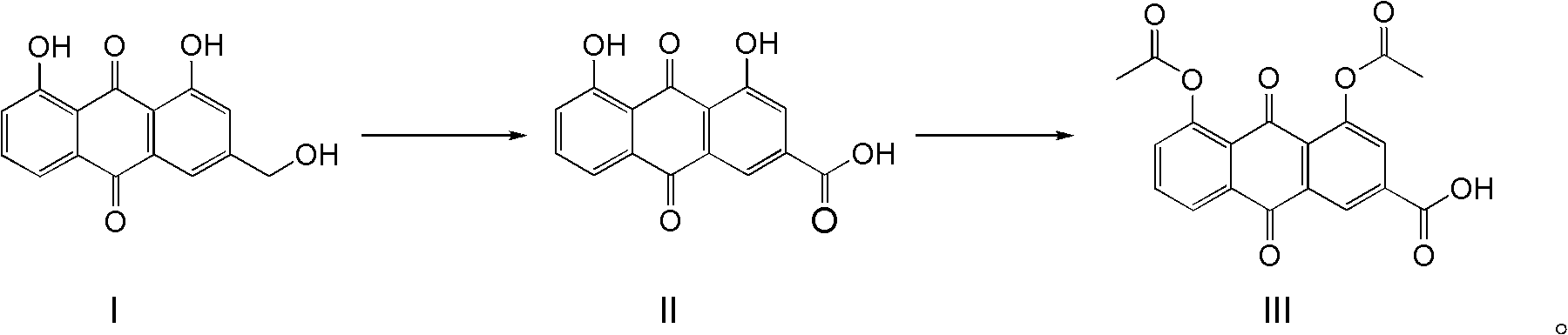
Synthetic process for diacerein
A synthesis process and a technology for diacerein are applied in the new synthesis process field of high-purity diacerein, and can solve the problems of high aloe-emodin residue, high cost, unfavorable industrialization, low product yield and purity, etc. Avoid the use of chromium-containing oxidants, the process is easy to control, and the product is easy to achieve
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Suspend aloe-emodin (100g) in N,N-dimethylformamide (1000mL), add sodium tungstate (1g), heat to about 80°C to completely dissolve aloe-emodin, and add to the reaction solution Slowly add 30wt% hydrogen peroxide (500mL) dropwise in the medium, and keep the reaction temperature at about 100°C until the hydrogen peroxide is added dropwise, and solids will gradually precipitate out. TLC or HPLC will track until the reaction is completely transformed. Starch potassium iodide reagent detects that there is no peroxide in the reaction system. The residue was cooled to room temperature, the precipitated solid was filtered, washed with water, and dried to obtain 101 g of rhein. The HPLC purity was 98.8%, and the yield was 96.2%.
Embodiment 2
[0030] Suspend aloe-emodin (100g) in N,N-dimethylformamide (300mL), add sodium tungstate (0.5g), heat to reflux to completely dissolve aloe-emodin, and slowly add to the reaction solution Add dropwise 30wt% hydrogen peroxide (200mL), maintain the reaction reflux, until the hydrogen peroxide is added dropwise, gradually solids are precipitated, TLC or HPLC is followed until the reaction conversion is complete, starch potassium iodide reagent detects that there is no peroxide residue in the reaction system, cool to room temperature, The precipitated solid was filtered, washed with water, and dried to obtain 101 g of rhein, with an HPLC purity of 98.5% and a yield of 96.2%.
Embodiment 3
[0032] Suspend aloe-emodin (100g) in N,N-dimethylacetamide (1000mL), add sodium tungstate (2g), heat to about 80°C to completely dissolve aloe-emodin, and add to the reaction solution Slowly add 30wt% hydrogen peroxide (500mL) dropwise in the medium, and keep the reaction temperature at about 100°C until the hydrogen peroxide is added dropwise, and solids will gradually precipitate out. TLC or HPLC will track until the reaction is completely transformed. Starch potassium iodide reagent detects that there is no peroxide in the reaction system. , cooled to room temperature, filtered the precipitated solid, washed with water, and dried to obtain 100 g of rhein, the HPLC purity was 98.9%, and the yield was 95.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com