1,3,4-oxadiazole derivative and preparation method and application thereof
A technology of oxadiazoles and derivatives, applied in oxadiazole derivatives and their preparation, the application field of fluorescent indicator in detecting intracellular calcium ions, can solve the self-fluorescence interference of biological samples, biological sample damage, It is not suitable for measuring cells or biological samples, etc., to achieve the effect of improving selectivity and sensitivity, reducing damage and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Synthesis of 2-(4-ethoxy)-phenyl)-5-(4-methyl)-phenyl)-1,3,4-oxadiazole:
[0039]
[0040] 4-Methylbenzoic acid (1.81g, 13.3mmol), add 2mLSOCl 2 , After stirring and refluxing for 3 h, the reaction mixture was concentrated under reduced pressure to obtain a yellow solution, namely p-toluoyl chloride. The cooled yellow solution was slowly added dropwise to 10 mL of chloroform solution containing 4-ethoxyphenylenehydrazine (2.18 g, 12.1 mmol) and 1.1 mL of pyridine, and the mixed solution was stirred and refluxed for 3 h, then concentrated under reduced pressure , cooled to obtain a solid mixture. The solid mixture was washed with water until neutral, and then dried. The dry mixture was recrystallized by adding hot ethanol to obtain 3.24 g of white crystals. After drying the white crystals in vacuo, add 10mL POCl 3 After reflux for 7h, it was concentrated under reduced pressure and cooled to obtain a solid mixture. The solid mixture was washed with NaAC (50%, ...
Embodiment 2
[0051] The lithium salt of 1,3,4-oxadiazole derivatives with the following structure can be obtained by alkaline hydrolysis of the 1,3,4-oxadiazole derivatives produced in Example 1 by LiOH.
[0052]
Embodiment 3
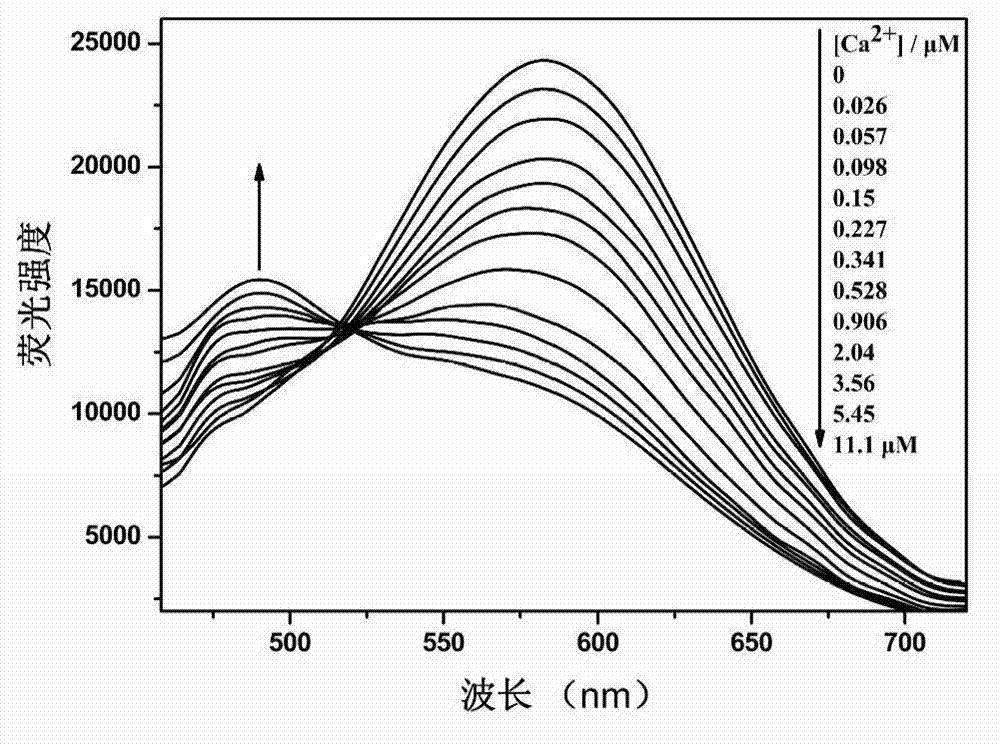
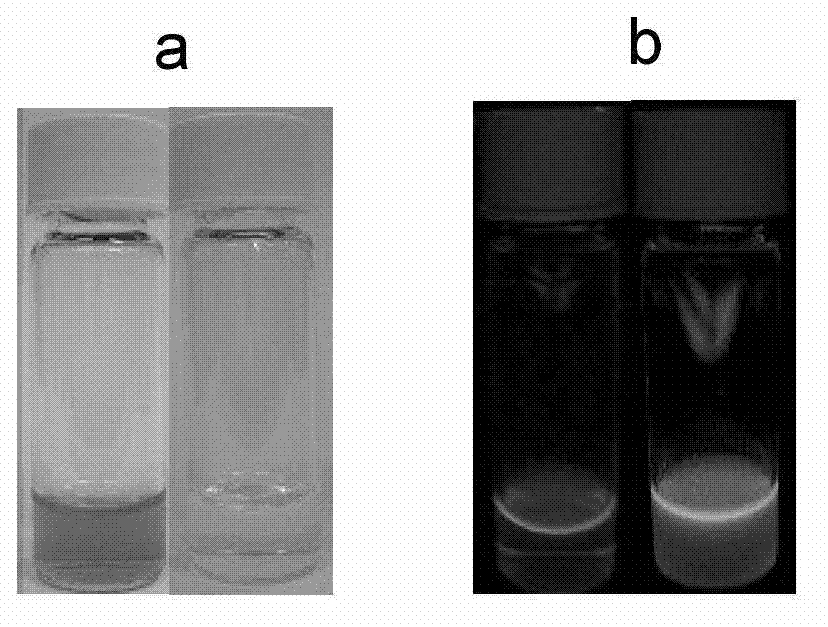
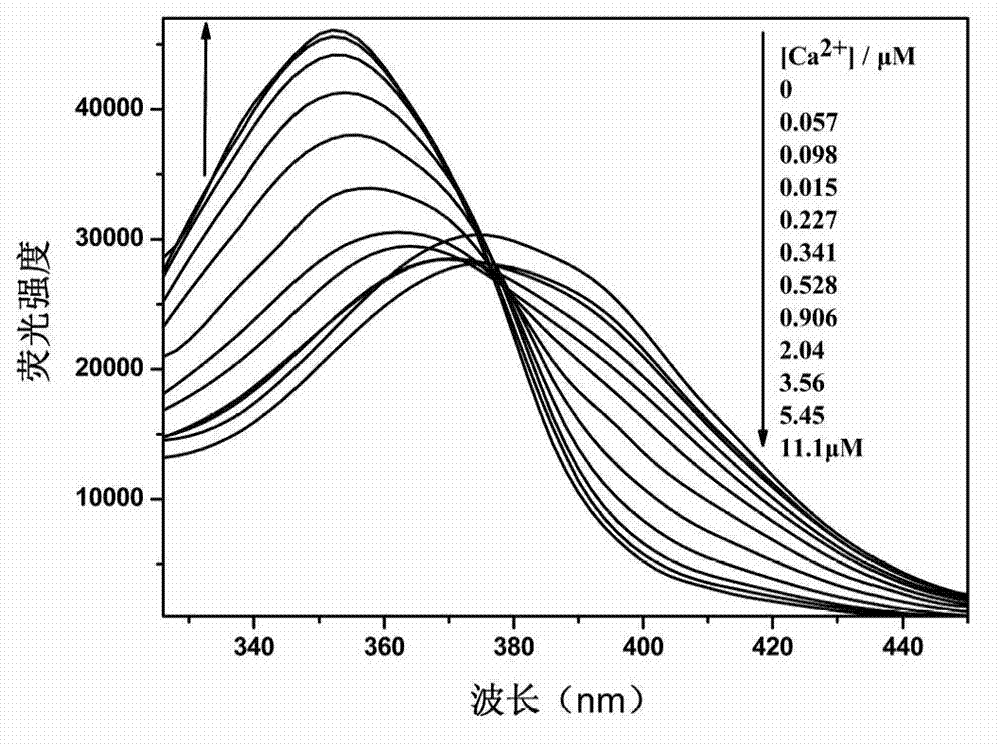
[0054] Ca 2+ The response group is 5,5'-dialdehyde-1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-ethyl tetraacetate, 2-(4-alkane Oxygen)-phenyl)-5-(4-bromomethyl)-phenyl)-1,3,4-oxadiazole reacts with a calcium ion responsive group in a molar ratio of 2:1, and the rest are the same as in the examples 1. Obtain 1,3,4-oxadiazole derivatives with the following structure.
[0055]
[0056] Yield 56%. Melting point: 60-62°C. The 1 H NMR (600MHz, CDCl 3 ) data are δ8.02(d, Ar-H, 4H), 7.80(d, Ar-H, 4H), 7.56(d, Ar-H, 4H), 7.38(d, Ar-H, 2H), 7.30( d, Ar-H, 2H), 7.04 (d, Ar-H, 4H, d, -CH=CH-, 2H), 6.90 (d, -CH=CH-, 2H), 6.85 (dd, Ar-H ,2H),4.60(s,-CH 2 -CH 2 -,4H),4.31(s,-CH 2 -,8H),4.10(q,-CH 2 -.12H),1.30(t,CH 3 -,18H).The 13 C NMR (600MHz, CDCl 3 ) data are δ170, 164, 159, 143, 138, 128, 121, 125, 115, 112, 69, 65, 61, 14.8 and 14.0. MALDI-TOF-MS: m / z: calcd for C 48 h 54 N 4 o 12 + :1168,found:1191.6904(M + +Na + ).Anal.Calcd for C 48 h 54 N 4 o 12 :C,67.79;H,5.86...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com