Method for determining 4-methoxyphenol in industrial acrylamide
A technology of p-hydroxyanisole and acrylamide, which is applied in the field of determination of p-hydroxyanisole, can solve the problems of strong corrosion of concentrated nitric acid, unsafe operation, and influence on the accuracy of polymerization inhibitors, and achieve high sensitivity and repeatability. Good performance, easy to popularize and apply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
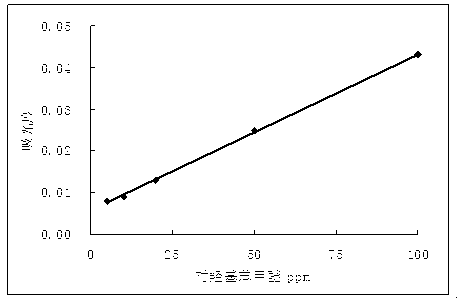
[0025] Example 1 The drawing of the standard curve
[0026] Making process to the standard curve of hydroxyzolitzo ether:
[0027] A. Called 0.01g of the standard product of hydroxybenzoic acid among the ice (accurate to 0.0001 g), placed in a 100ml capacity bottle with 50 ml of methampionic acid.Scale;
[0028] B. Take the above solution of 0.5ml, 1ml, 2 ml, 5ml, and 10ml of the above solution, inject the 100ml capacity bottle in turn, set the icylic acid, dilute to 0.5ppm, 1ppm, 2PPM, 5PPM, and 10PPM.
[0029] C. The above standard solution is 10ml in order. At this time, the content of hydroxyl benzene ether in the standard solution is 5ppm, 10ppm, 20ppm, 50ppm, and 100PPM, respectively, and placed in a capacity bottle of 50ml with 20 ml of methamphetamine.Sodium nitrite solution, and dilute it with methamphetamine to the scale, fully mix, and place it for 10 minutes;
[0030] D. Taking icedetic acid as a blank, measure the inhalation of each standard solution under the wavelen...
Embodiment 2
[0034] Example 2 Sample measurement
[0035] Mercy of acrylamide samples containing hydroxythexyl ether, which are divided into two categories: solid powder and water agent: solid powder and water:
[0037] It is said that about 3g of acrylamide powder containing hydroxybenzenyl ether is dissolved in a capacity bottle of 50ml of methamphetamic acid with 20ml of icycety acid, add 1 mL of sodium nitrite solution, and dilute it with methamphetamine to the scale, mix it fully, place 10 min 10 min., Taking Icephinotic acid as a blank, determine its absorbance at 420Nm, and find the content of the hydroxybenzo ether (C) corresponding to the working curve of the working curve, calculate the content of the hydroxyzenyl ether in acrylamide according to the following formulaThe
[0038] The content of the hydroxyzen benzene ether (PPM) = C / X;
[0039] In the formula, the content of the C-working curve to the hydroxybenzoethyl ether, μg; the quality of X-acrylamide, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com