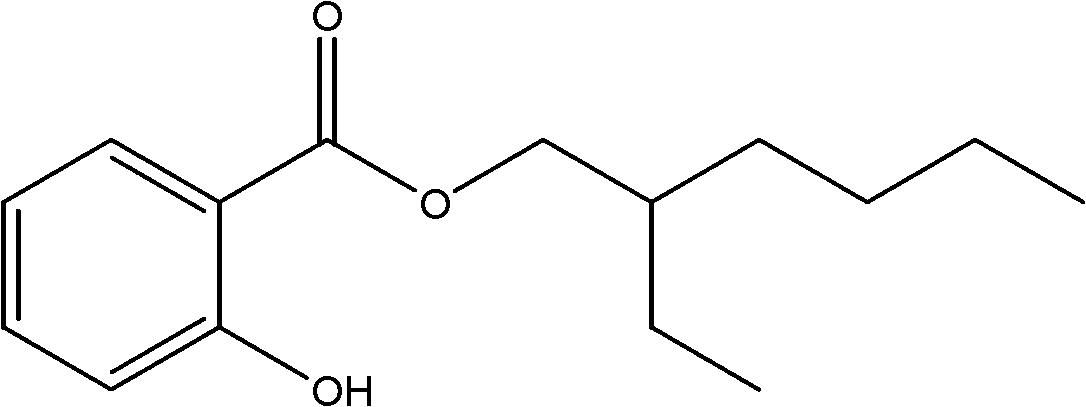
Synthesis method of 2-ethylhexyl salicylate
A technology of isooctyl salicylate and a synthesis method, which is applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc., can solve problems such as no literature reports, and achieves easy operation, simple steps, Highly active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] 1) Preparation of tungstic acid: Stir continuously in sodium tungstate and add to 1mol / L sulfuric acid solution, age and filter; wash with deionized water, dry at 100°C to obtain H 2 WO 4 solid, ground into powder;
[0024] 2) Amorphous TiO 2 ·nH 2 Preparation of O: TiCl 4 Dissolve in deionized water with continuous stirring in an ice bath, add concentrated ammonia water dropwise for hydrolysis, age and filter; wash with deionized water until Cl-free - , and then dried at 110 °C to obtain amorphous TiO 2 ·nH 2 O, ground into powder;
[0025] 3) Solid superacid SO 4 2- / TiO 2 -WO 3 Preparation: Take the H prepared above 2 WO 4 Powder and Amorphous TiO 2 ·nH 2 O powder mixed in a certain proportion (H 2 WO 4 The mass fraction is 10%), impregnated with dilute sulfuric acid solution, filtered, baked at 110°C for 3 hours, and then calcined at higher than 500°C to obtain an acid catalyst.
Embodiment 1
[0028] Weigh 33g Na 2 WO 4 2H 2 O, add to 50mL 1mol / L sulfuric acid solution deionized water with constant stirring, filter after aging. After washing with deionized water and drying at 100 °C, a yellow solid H 2 WO 4 , ground into powder; at the same time, measure 25mL TiCl 4 Dissolve in deionized water with continuous stirring in an ice bath, dropwise add concentrated ammonia water to hydrolyze until the solution is alkaline, filter after aging; wash with deionized water until Cl-free - , and then dried at 110 °C to obtain amorphous TiO 2 ·nH 2 O, ground into powder; weigh 2g H 2 WO 4 and 18g amorphous TiO 2 ·nH 2 O powder, mixed evenly, impregnated with 1mol / L sulfuric acid solution for 3h, filtered and baked at 110°C for 3h, then roasted at higher than 500°C to obtain solid super acid SO 4 2- / TiO 2 -WO 3 catalyst.
[0029] Add (13.8g, 0.1mol) salicylic acid and (50mL, 0.3mol) isooctyl alcohol into a 100mL three-necked flask, stir well, then add 2g of homema...
Embodiment 2
[0033] Add (13.8g, 0.1mol) salicylic acid and (50mL, 0.3mol) isooctyl alcohol into a 100mL three-necked flask, stir well, then add the solid superacid SO which was filtered and dried in Example 1 4 2- / TiO 2 -WO 3 Catalyst, heated to reflux at 190°C, reacted for 5h, stopped heating, cooled to room temperature, and filtered out the catalyst. After the filtrate was washed with hot water at 65°C, it was washed with saturated NaHCO 3 Wash until neutral, then wash with hot water. Separate the ester layer with anhydrous MgSO 4 Dry, filter with suction, distill under reduced pressure, collect the fraction with a boiling point of 84~86°C, and recover the excess solvent; collect the fraction at 172~174°C / 1.0kPa to obtain 20.5g of isooctyl salicylate product, with a yield of 81.9% ( as salicylic acid).
[0034] of the compound 1 The HNMR data are as follows:
[0035] 1 HNMR(DMSO)δ: 0.833~0.928(m, 6H, CH 3 ), 1.279~1.447 (m, 8H, CH 2 ), 1.684~1.714(m, 1H, CH), 4.242~4.255(d, 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com