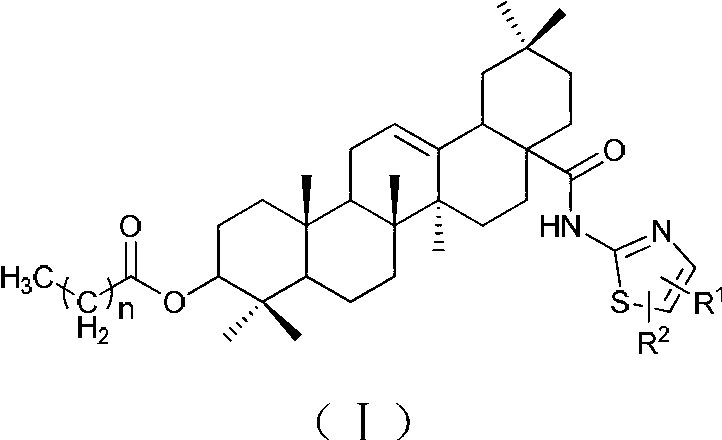
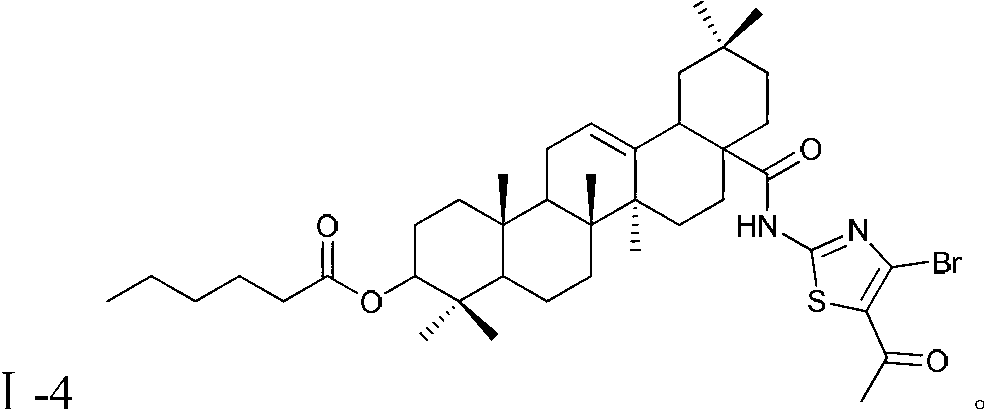
Oleanolic acid derivatives, and preparation method and application thereof
A compound and pharmaceutical technology, applied in the field of medicine, can solve the problems of large gastrointestinal damage, liver toxicity, weight gain, etc., and achieve a significant effect of lowering blood sugar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Reference Example 1: Synthesis of Intermediate II
[0044]
[0045] Add 4.57g (0.01mol) of oleanolic acid into a reaction flask equipped with a stirring, condenser, and thermometer, then add 20mL of thionyl chloride to dissolve it, heat up to reflux reaction, and stop the reaction after no acid gas is released. Evaporate thionyl chloride under reduced pressure to obtain intermediate Ⅱ.
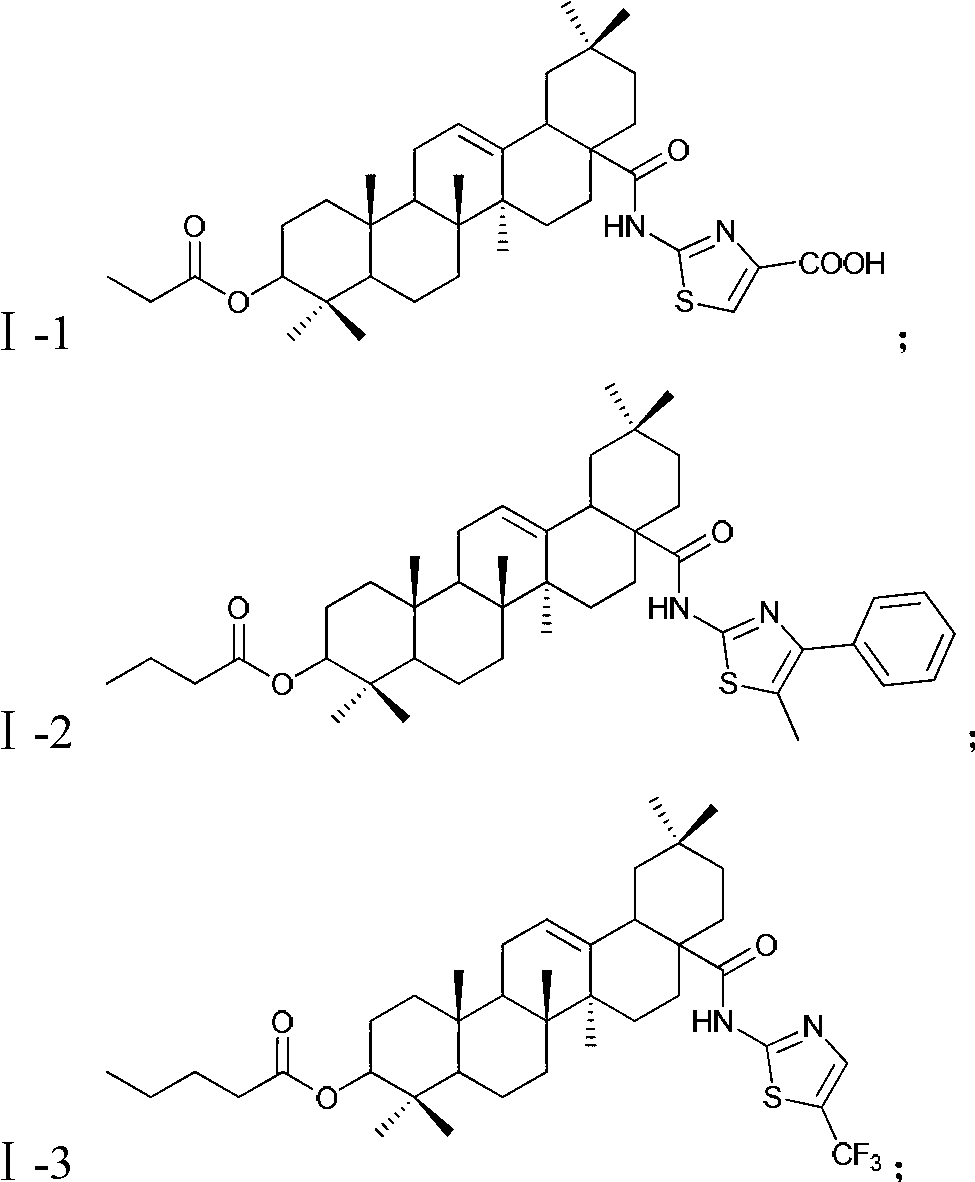
[0046] Example 1: Synthesis of Compound Ⅰ-1
[0047]
[0048] Add 4.75g of intermediate II to a reaction flask equipped with a stirring, condenser and thermometer, add 20mL of propionic acid to dissolve it, and add an appropriate amount of concentrated sulfuric acid, reflux reaction, and TLC to control the reaction process. After the reaction, the reaction solution was cooled to -10°C, and triethylamine was added to neutralize the acid in the reaction system until the reaction solution was alkaline. Add 30 mL of dichloromethane, and add 1.44 g (0.01 mol) of 2-amino-4-carboxyt...
Embodiment 2
[0049] Example 2: Synthesis of Intermediate Ⅰ-2
[0050]
[0051] Add 4.75g of Intermediate II into a reaction flask equipped with a stirring, condenser and thermometer, add 20mL of butyric acid to dissolve it, and add an appropriate amount of concentrated sulfuric acid, and reflux the reaction. TLC controls the reaction process. After the reaction, the reaction solution was cooled to -5°C, and pyridine was added to neutralize the acid in the reaction system until the reaction solution was alkaline. Add 30 mL of chloroform, and add 1.90 g (0.01 mol) of 2-amino-4-phenyl-5-methylthiazole in batches under stirring. After the addition, the temperature is raised to reflux and the reaction is continued for 1.5 h (the plate shows that the reaction is complete). Wash the reaction solution with 3×30mL water, separate the chloroform layer, fully dry it with anhydrous sodium sulfate, filter, and evaporate the chloroform under reduced pressure to obtain a light yellow solid product, ...
Embodiment 3
[0052] Example 3: Synthesis of Intermediate Ⅰ-3
[0053]
[0054] Referring to the method of Reference Examples 1 and 2, the esterification reaction occurred with intermediate II and n-valeric acid. After the reaction, the reaction solution was cooled to 0°C, pyridine and dichloromethane were added, and 2-amino-5-trifluoromethylthiazole was added in batches under stirring. After the reaction, the reaction solution was washed with water, and the chloroform layer was separated. Fully dry with anhydrous sodium sulfate, filter, and evaporate chloroform under reduced pressure to obtain a yellow solid product, which is refined with ethyl acetate-petroleum ether to obtain 2.4 g of a white solid product (HPLC: 99.6%), yield 34.7% . Rf=0.44[single point, developer: v(petroleum ether):v(ethyl acetate)=4:1]. MS, m / Z: 691.4 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com