Zinc ferrite anode material of lithium ion battery and preparation method and application thereof
A lithium-ion battery and negative electrode material technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of increased irreversible capacity loss, poor high-rate charge-discharge performance, and low initial efficiency, achieving excellent electrochemical performance, High charge and discharge efficiency and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
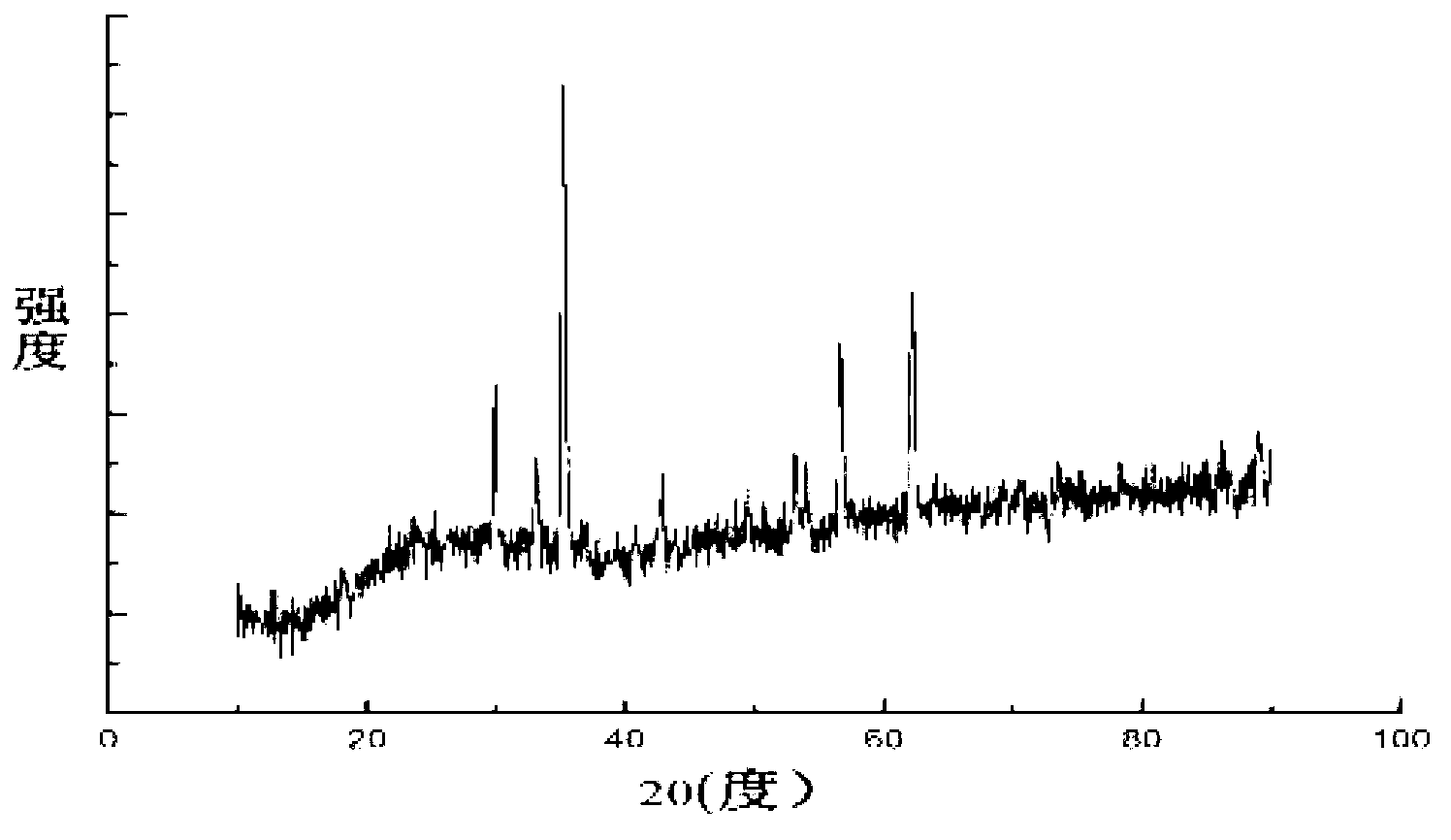
[0046] The zinc chloride of 2.18g, the iron chloride of 5.89g are mixed and dissolved in the ethylene glycol solution of 100ml respectively, wherein the molar concentration of zinc chloride is 0.16mol / L, and the molar concentration of ferric chloride is 0.32mol / L L, the molar ratio of zinc chloride to ferric chloride is 1:2, and 4.62g of ammonium acetate is added as a protective agent at the same time, and the resulting mixed solution is stirred for 1.5h. Then pour the uniformly mixed solution into a 200ml high-pressure sealed tank, heat at 180°C for 24 hours to obtain a black precipitate, centrifuge to filter out the black precipitate, wash with absolute ethanol and deionized water alternately for 5 times, and dry at 50°C for 6 hours. The final product obtained was detected by XRD pattern, and the detection results were as follows: figure 1 As shown, the three strong peaks of the spectrum are consistent with the zinc ferrite standard card JCPDS no.22-1012, indicating that the...
Embodiment 2
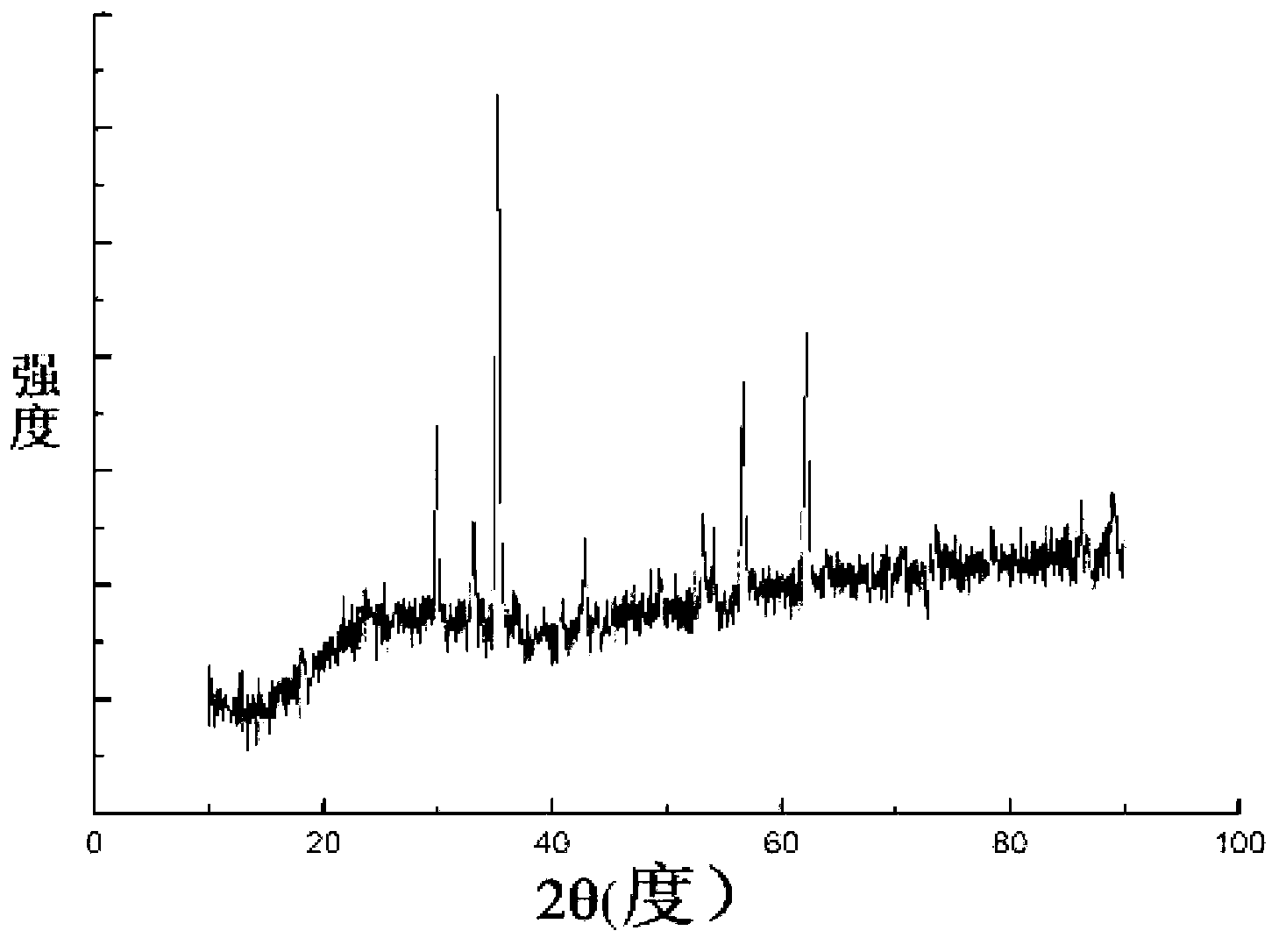
[0049]The zinc chloride of 2.18g, the iron chloride of 5.89g are mixed and dissolved in the ethylene glycol solution of 100ml respectively, wherein the molar concentration of zinc chloride is 0.16mol / L, and the molar concentration of ferric chloride is 0.32mol / L L, the molar ratio of zinc chloride to ferric chloride is 1:2, and 4.62g of ammonium acetate is added as a protective agent at the same time, and the resulting mixed solution is stirred for 1.5h. Then pour the uniformly mixed solution into a 200ml high-pressure sealed tank, heat at 180°C for 48 hours to obtain a black precipitate, centrifuge to filter out the black precipitate, rinse with absolute ethanol and deionized water alternately for 5 times, and dry at 50°C for 6 hours. The final product obtained was detected by XRD pattern, and the detection results were as follows: figure 2 As shown, the three strong peaks of the spectrum are consistent with the zinc ferrite standard card JCPDS no.22-1012, indicating that th...
Embodiment 3
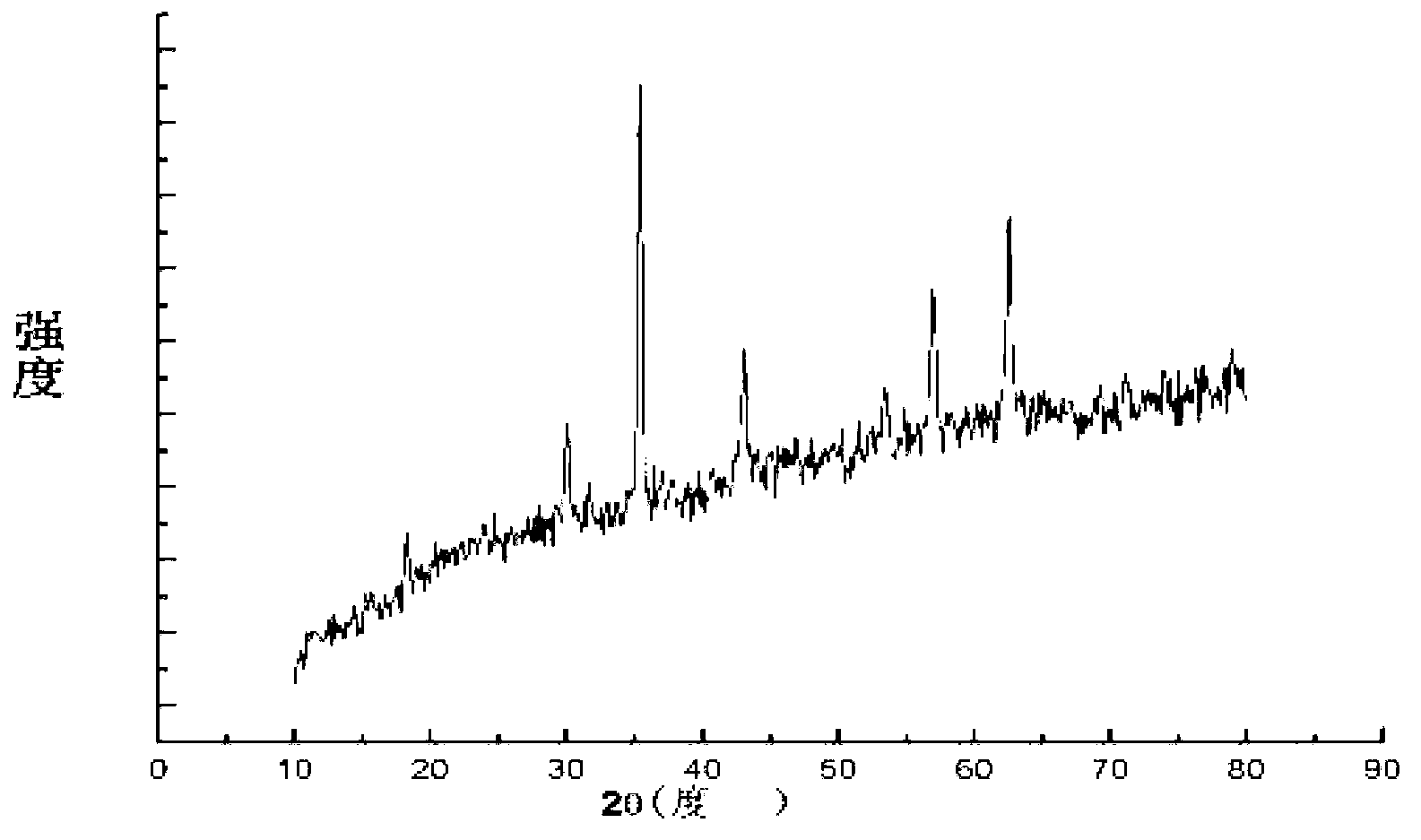
[0052] The zinc chloride of 2.18g, the iron chloride of 5.89g are mixed and dissolved in the ethylene glycol solution of 100ml respectively, wherein the molar concentration of zinc chloride is 0.16mol / L, and the molar concentration of ferric chloride is 0.32mol / L L, the molar ratio of zinc chloride to ferric chloride is 1:2, and 4.62g of ammonium acetate is added as a protective agent at the same time, and the resulting mixed solution is stirred for 1.5h. Then pour the uniformly mixed solution into a 200ml high-pressure sealed tank, heat at 200°C for 48 hours to obtain a black precipitate, centrifuge to filter out the black precipitate, wash with absolute ethanol and deionized water alternately for 5 times, and dry at 50°C for 6 hours. The final product obtained was detected by XRD pattern, and the detection results were as follows: image 3 As shown, the three strong peaks of the spectrum are consistent with the zinc ferrite standard card JCPDS no.22-1012, and there are no di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com