Azido-containing naphthalimide compound and applications thereof in detecting hydrogen sulfide
A nitrogen-based naphthalimide and compound technology, which is applied to the application field of azide-containing naphthalimide compounds and hydrogen sulfide detection, can solve problems such as unfavorable detection of hydrogen sulfide content, achieve the expansion of linear detection range, change Response time, fast response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] Synthesis of N-(n-butyl)-4-azido-1,8-naphthalimide (compound 1):
[0031] (1) Add 50mL of ethanol, 5.54g of 4-bromo-1,8-naphthalene anhydride (20mmol) and 2.5g of n-butylamine (34.3mmol) in a 100mL three-necked flask, heat the mixture under reflux for 4 hours, and remove solvent. The reaction product was recrystallized twice with absolute ethanol to obtain a light yellow solid with a yield of 85%.
[0032] (2) Weigh 0.50 g of the product synthesized by step (1) and 0.20 g of sodium azide, mix and dissolve it in 20 mL of N,N-methylformamide (DMF), and heat the mixture to 80°C under nitrogen protection. For 12 hours, the reaction was followed by thin-plate chromatography until the reaction was complete. After the reaction, the solution was poured into 100 mL of deionized water, and the above solution was extracted three times with dichloromethane, the organic phases were combined, and the solvent was distilled off under pressure. The resulting solid was sep...
Embodiment 2
[0034] Synthesis of N-(diethylene glycol)-4-azido-1,8-naphthalimide (compound 2)
[0035] Except that diglycolamine is used to replace n-butylamine, other synthesis and purification methods are the same as in Example 1. The product (compound 2) was obtained with a yield of 55%.
[0036] 1 H-NMR (400MHz, CDCl 3 ): 8.64(d, J=7.2Hz, 1H, ArH), 8.58(d, J=8.0Hz, 1H, Ar-H), 8.44(d, J=8.4Hz, 1H, Ar-H), 7.75( t, J=7.6Hz, 1H, Ar-H), 7.47(d, J=8.0Hz, 1H, Ar-H), 4.44(t, J=5.6Hz, 2H, CH 2 ), 3.86(t, J=5.6Hz, 2H, CH 2 ), 3.68 (m, 4H, 2CH 2 ); LC-MS (ESI): m / z 327.31 (M+H) + , calculated 327.40
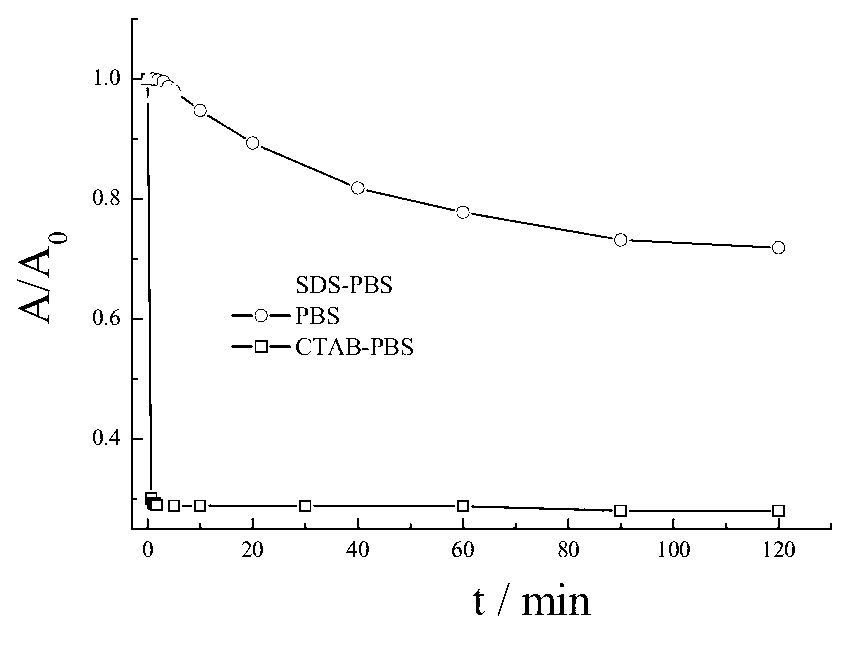
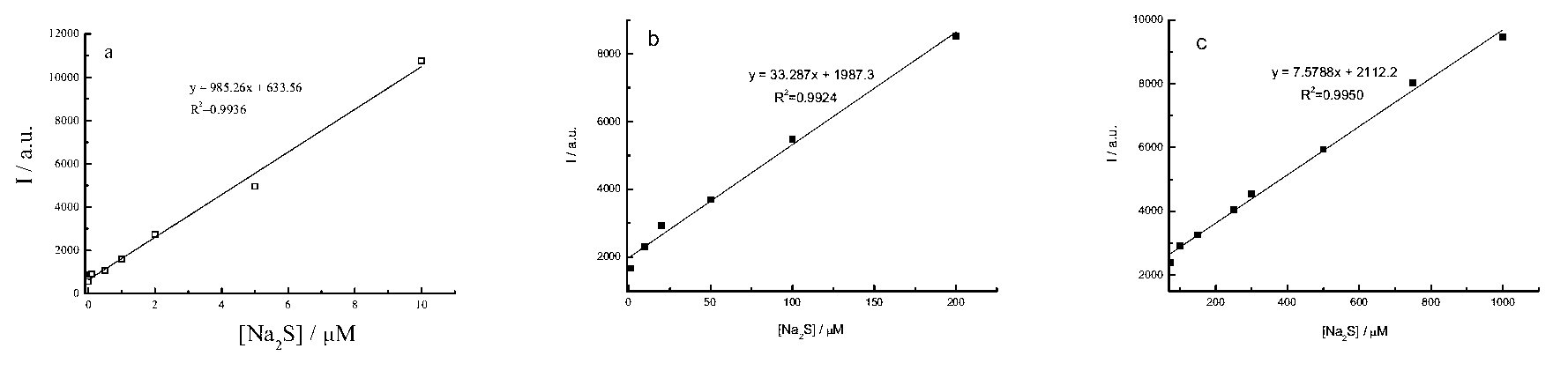
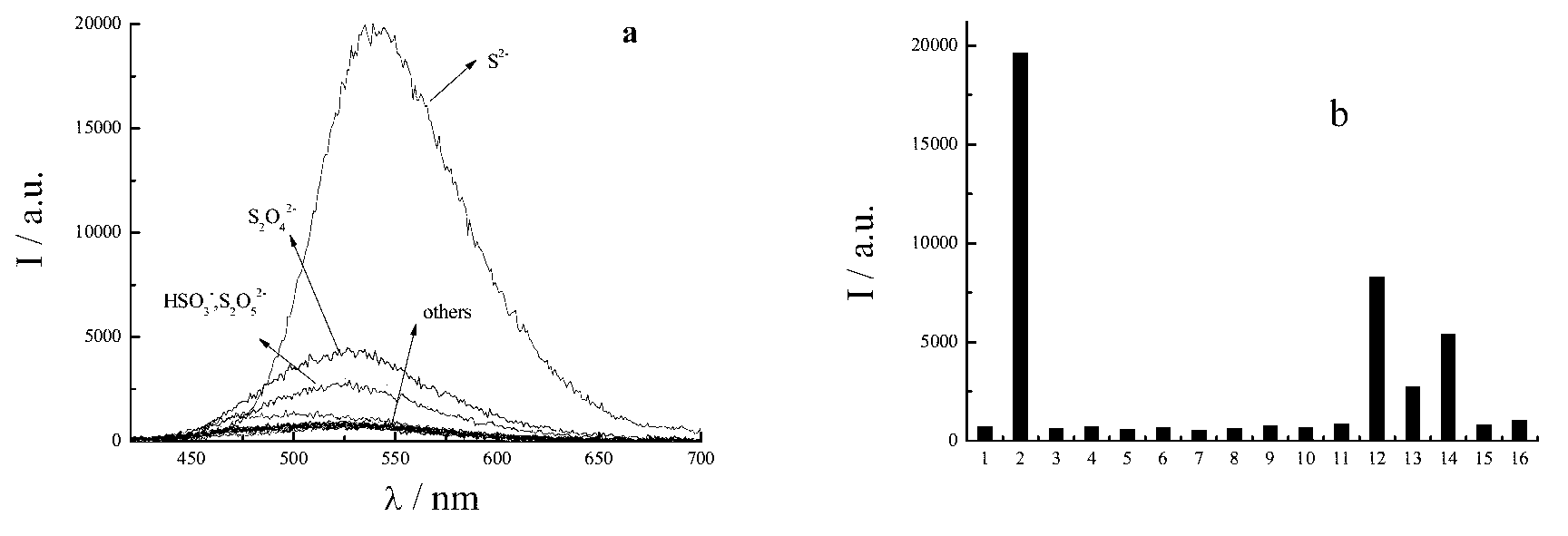
[0037] Determination of hydrogen sulfide in buffer solution and surfactant system:
[0038] Accurately weigh a certain amount of compound 1 or 2 and dissolve it in 10mL ethanol to prepare a 1mM stock solution and store it in the dark; accurately weigh a certain amount of Na 2 Dissolve S in 10 mL of water to make 1 mM Na 2 S stock solution. Accurately pipette 100 μL stock solution of compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com