Method for extracting vanadium from vanadium-containing highly concentrated sulfuric acid solution and application of extracting agent
A sulfuric acid solution, high-concentration technology, applied in the field of wet extraction of vanadium and vanadium, can solve the problems of huge consumption of acid and alkali, high consumption of sulfuric acid, complicated process, etc. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
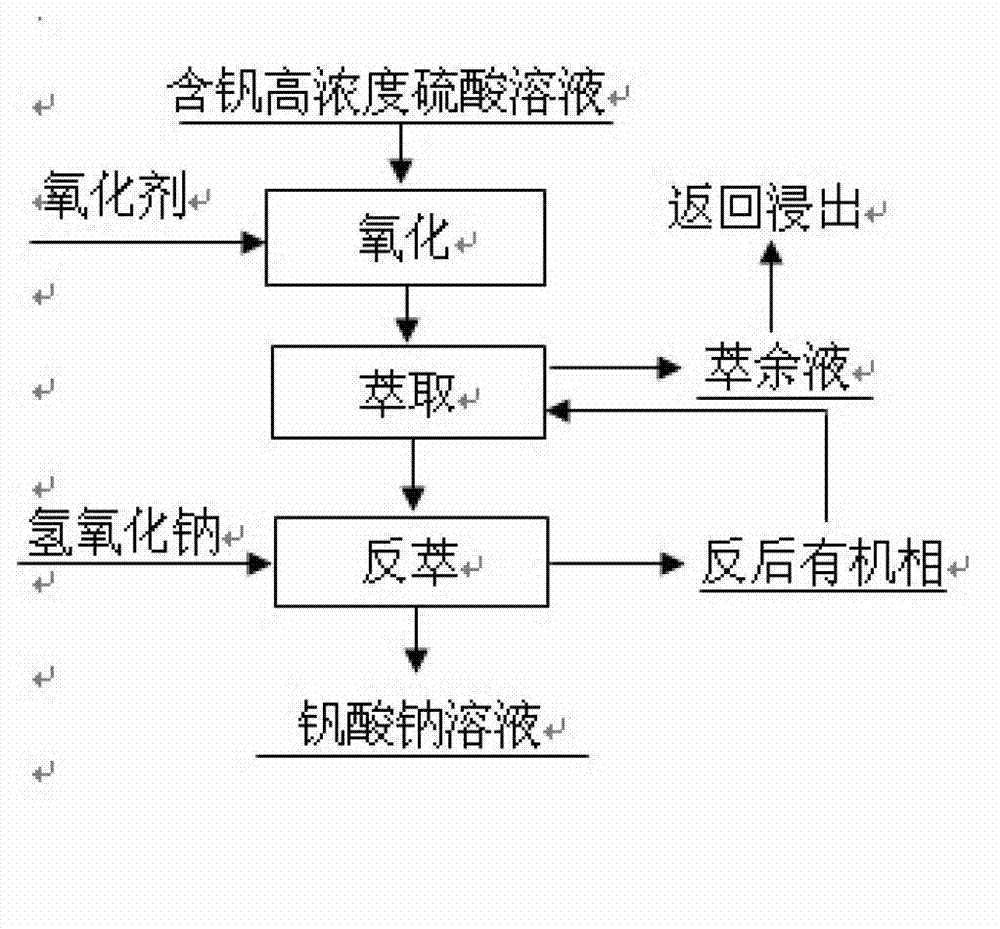
[0044] according to figure 1 The process flow is operated;
[0045] The feed liquid is stone coal mine sulfuric acid leaching solution, containing 5.75g / L of vanadium and 1.5mol / L of free sulfuric acid;
[0046] The organic phase is 10% extractant + sulfonated kerosene;
[0047] First, add hydrogen peroxide to the leaching solution at room temperature, add 8 mL of 22% hydrogen peroxide for each leaching solution with a potential of 445 mV, stir for 30 minutes, and finally the potential of the material solution is 850 mV;
[0048] A separatory funnel was used for single-stage extraction, the extraction ratio (O / A) = 1 / 2, the extraction time was 10 minutes, the temperature was 30°C, and the phase separation was 3 minutes. The results are shown in Table 1.
[0049] Table 1 Extraction experiment results
[0050] Element
[0051] The results show that during the extraction process, single-stage extraction of vanadium can achieve an extraction rate of 83.8%, and almost...
Embodiment 2
[0053] according to figure 1 The process flow is operated;
[0054] The feed liquid is sulfuric acid leaching solution of stone coal mine, containing vanadium 7.00g / L, [H + ] is 1.55mol / L;
[0055] The organic phase is 10% extractant + sulfonated kerosene;
[0056] First, add different amounts of sodium chlorate to the leaching solution at room temperature, stir for 30 minutes, and obtain four kinds of oxidizing feed solutions with potentials of 780mV, 960mV, 1070mV, and 1140mV respectively;
[0057] A separatory funnel was used for single-stage extraction, the extraction ratio (O / A)=1 / 1, the extraction time was 10 minutes, the temperature was 30°C, and the phase separation was 10 minutes. The results are shown in Table 2.
[0058] Extraction experiment results under different material-liquid potential conditions in table 2
[0059] electric potential
[0060] The results show that when the potential of the vanadium-containing material liquid is above 850mV, the ex...
Embodiment 3
[0062] according to figure 1 The process flow is operated;
[0063] The feed liquid is sulfuric acid leaching solution of stone coal mine, containing 7.00g / L of vanadium and potential of 445mV;
[0064] The organic phase is 10% extractant + sulfonated kerosene;
[0065] First add sodium chlorate to the leaching solution at room temperature, add 3.3g sodium chlorate per liter of feed solution, adjust the potential to 885mV, and then add different amounts of solid sodium hydroxide or 98% sulfuric acid to obtain 7 kinds of different acidity Liquid;
[0066] Separated funnels were used for single-stage extraction, the extraction ratio (O / A) = 1 / 2, the extraction time was 10 minutes, the temperature was 30°C, and the phase separation was 10 minutes. The results are shown in Table 3.
[0067] Table 3 Extraction experiment results under different feed liquid acidity conditions
[0068] Acidity [H + ] (mol / L)
[0069] Extraction rate of magnesium (%)
[0070]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com