Pharmaceutical for treating pneumonia, as well as preparation method and quality control method of pharmaceutical formulation
A technology of traditional Chinese medicine preparations and pneumonia, applied in the field of preparation of medicines and their preparations, can solve problems affecting the absorption and utilization of active ingredients of medicines, slow release of active ingredients of raw materials, and inability to ensure stable and uniform quality of this product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
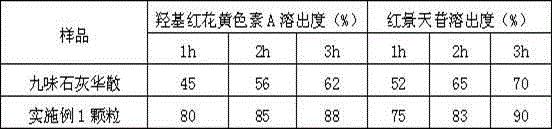
experiment example 1
[0044] Experimental example 1: Comparison of different extraction methods
[0045] A. Water extraction:
[0046] (1) Take safflower and sandalwood according to the composition ratio of raw materials, add 8 times the weight of water, use steam distillation method, extract volatile oil for 4 hours, collect volatile oil, filter the medicinal liquid, and obtain extract A' and medicinal residue A;
[0047] Add the collected volatile oil into 4% β-cyclodextrin aqueous solution by weight and volume, the volume-to-weight ratio of volatile oil to β-cyclodextrin is 1ml: 4g, under stirring conditions, keep the temperature at 50°C, stir for 4h, 0°C-4 Refrigerate at ℃ overnight, filter with suction, and take the precipitate to obtain the clathrate B of volatile oil;
[0048] (2) Take a total of 6 medicinal materials according to the composition ratio of raw materials: Travertine, Rhodiola, Bangga, Licorice (peeled), Alpine horseradish, and Honglian, and mix with the medicinal residue A af...
experiment example 2
[0059] Experimental example 2: Selection of different excipients
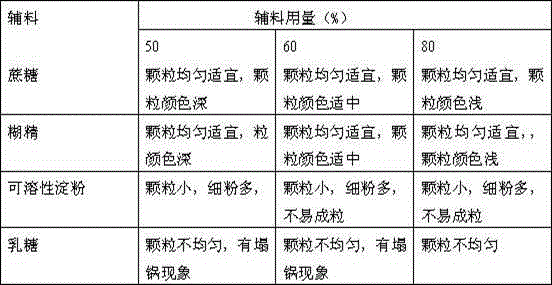
[0060] Take artificial bezoar and the volatile oil clathrate B obtained by the water extraction method of the above-mentioned experimental example 1 according to the composition ratio of the raw material drug, add it to the liquid extract C obtained by the water extraction method of the above-mentioned experimental example 1, mix well with a colloid mill, and add sucrose respectively , dextrin, soluble starch, lactose as auxiliary materials, one-step granulation with boiling spray to prepare granules;
[0061] Table 3 Effects of different excipients and dosages on granules
[0062]
[0063] Note: The amount of auxiliary materials refers to the weight percentage of the finished granules
[0064] The results show that it is more suitable to use sucrose and dextrin as auxiliary materials, and the obtained particles are uniform and suitable, and the dosage can be 50%-80%.
experiment example 3
[0065] Experimental example 3: Selection of different granulation processes
[0066] Prepare volatile oil clathrate B and liquid extract C according to the method of Example 1, and mix them with artificial bezoar, use dextrin as an auxiliary material, and the dosage is 60% of the finished granules, and adopt one-step granulation and wet granulation respectively to prepare Granules, the resulting granules measure the loss of active ingredient;
[0067] Table 4 Effect of different granulation methods on active ingredients
[0068] Granulation process Loss rate of hydroxysafflower yellow A (%) Loss rate of salidroside (%) one step granulation 1.6 2.1 wet granulation 13.8 7.5
[0069] The results showed that there was almost no loss of active ingredients in one-step granulation, but the loss of active ingredients in wet granulation, especially hydroxysafflower yellow A, was more serious, so it was determined to adopt one-step granula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com