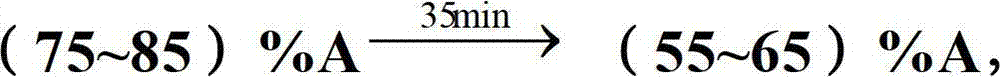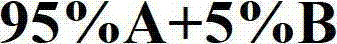Purification method of lixisenatide
A technology of lixisenatide and purification method, applied in the field of purification of lixisenatide, can solve problems such as restricting industrialization, and achieve the effects of easy operation, improved yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
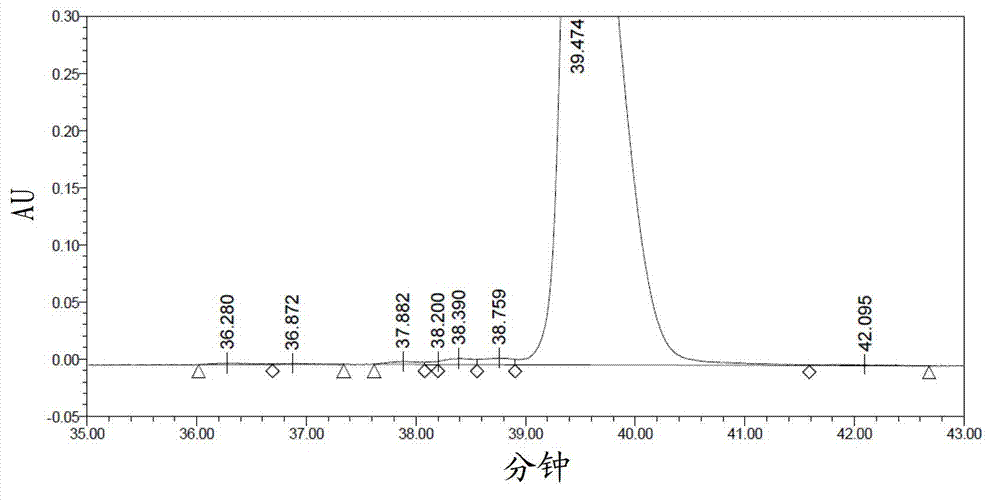
[0038] Dissolve 4.0 g of lixisenatide crude peptide (including 1.08 g of lixisenatide) in 200 ml of purified water, filter, and collect the filtrate for later use.
[0039] Purification chromatography conditions:
[0040] High performance liquid chromatography model: Waters 2545
[0041] Chromatographic column: 50×250mm, with phenylsilane bonded silica gel inside as the stationary phase filler, the particle size of the filler is 10μm.
[0042] Flow rate: 80ml / min.
[0043] Detection wavelength: 280nm.
[0044] Mobile phase A: 10mM D-ammonium tartrate and 30mM ammonium dihydrogen phosphate in 20% methanol / 80% aqueous solution (v / v), adjust pH to 2.0 with phosphoric acid.
[0045] Preparation process of mobile phase A: Weigh 18.4g D-ammonium tartrate and 34.5g ammonium dihydrogen phosphate, dissolve in appropriate amount of purified water, filter through a 0.45μm filter membrane, collect all the filtrate into a 10L serum bottle, add 2L chromatographically pure methanol Purif...
Embodiment 2
[0065] Dissolve 6.0 g of lixisenatide crude peptide (including 1.62 g of lixisenatide) in 300 ml of purified water, filter, and collect the filtrate for later use.
[0066] Purification chromatography conditions:
[0067] High performance liquid chromatography model: Waters 2545
[0068] Chromatographic column: 50×250mm, with phenylsilane bonded silica gel inside as the stationary phase filler, the particle size of the filler is 10μm.
[0069] Flow rate: 80ml / min.
[0070] Detection wavelength: 280nm.
[0071] Mobile phase A: 40mM D-sodium tartrate and 20mM ammonium dihydrogen phosphate in 15% methanol / 85% aqueous solution (v / v), adjust the pH to 3.0 with phosphoric acid.
[0072] Preparation process of mobile phase A: weigh 92.0g sodium D-tartrate and 23.0g ammonium dihydrogen phosphate, dissolve in appropriate amount of purified water and filter through a 0.45μm filter membrane, collect all the filtrate into a 10L serum bottle, add 1.5L chromatographically pure methanol ...
Embodiment 3
[0090] Dissolve 6.0 g of lixisenatide crude peptide (including 1.62 g of lixisenatide) in 300 ml of purified water, filter, and collect the filtrate for later use.
[0091] Purification chromatography conditions:
[0092] High performance liquid chromatography model: Waters 2545
[0093] Chromatographic column: 50×250mm, with phenylsilane bonded silica gel inside as the stationary phase filler, the particle size of the filler is 10μm.
[0094] Flow rate: 80ml / min.
[0095] Detection wavelength: 280nm.
[0096] Mobile phase A: 30mM D-potassium tartrate and 40mM potassium dihydrogen phosphate in 10% methanol / 90% aqueous solution (v / v), adjust the pH to 2.5 with phosphoric acid.
[0097] Preparation process of mobile phase A: Weigh 56.1g D-potassium tartrate and 54.4g potassium dihydrogen phosphate, dissolve in appropriate amount of purified water and filter through a 0.45μm filter membrane, collect all the filtrate into a 10L serum bottle, add 1.0L chromatographically pure me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com