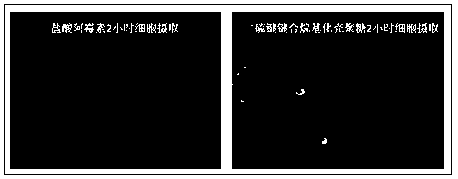
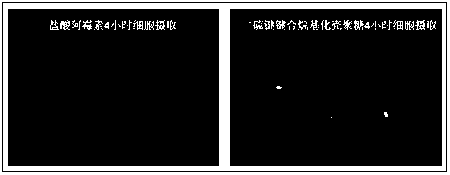
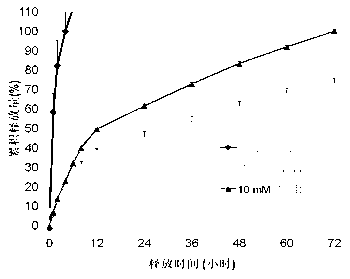
Glutathione sensitive alkylated chitosan and preparation and application thereof
A technology of chitosan and chitosan, which is applied in the application field of disulfide bonded alkylated chitosan as a drug carrier material, can solve the problem of affecting the curative effect and increasing the preparation of chitosan microparticles and nanoparticles Difficulty, large molecular weight, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1. Preparation of low molecular weight chitosan
[0025] Take commercially available chitosan with a molecular weight of 350kDa (70% degree of deacetylation), and stir it under aqueous hydrochloric acid solution at 55°C for 2 hours to fully swell the chitosan, then add 5%wt. cellulase to degrade it to The degree of degradation of chitosan was controlled by the viscosity method. The obtained chitosan degradation liquid is filtered to remove impurities, and the ultrafiltration membranes with molecular weights of 50 kDa and 100 kDa are used for ultrafiltration classification. The ultrafiltrate with a molecular weight between 50kDa and 100kDa was freeze-dried to obtain a low molecular weight chitosan with a deacetylation degree of 90% and a molecular weight of 75kDa.
[0026] 2. Preparation of disulfide bonded alkylated chitosan and determination of its physical and chemical properties
[0027] Precisely weigh 130 mg of the cross-linking agent 3,3'-dithiodipropionic acid ...
Embodiment 2
[0038] 1. Preparation of low molecular weight chitosan
[0039] Take commercially available chitosan with a molecular weight of 350kDa (90% degree of deacetylation), and stir it for 2 hours at 55°C and pH 5.0 to fully swell the chitosan, then use the ratio of cellulase to chitosan as 0.5: 100 (w / w) cellulase was added to degrade chitosan. The degree of degradation of chitosan was controlled by viscosity method. The obtained chitosan degradation solution is filtered to remove impurities, and the ultrafiltration membranes with molecular weights of 1KDa and 3KDa are used for ultrafiltration classification. The fractionated ultrafiltrate was freeze-dried to obtain chitosan with a deacetylation degree of 95% and a molecular weight of 1.2KDa.
[0040] 2. Preparation of disulfide bonded alkylated chitosan and determination of its physical and chemical properties
[0041] Accurately weigh 590 mg of the cross-linking agent dithiodisuccinopropionic acid in the prescribed amount, diss...
Embodiment 3
[0049] 1. Preparation of low molecular weight chitosan
[0050] Take commercially available chitosan with a molecular weight of 350kDa (70% degree of deacetylation), and stir it for 2 hours at 55°C with hydrochloric acid water pH 5.0 solution to fully swell the chitosan, then add 5%wt. cellulase to degrade it , to control the degradation degree of chitosan by viscosity method. The resulting chitosan degradation solution was filtered to remove impurities, and ultrafiltration was performed using ultrafiltration membranes with molecular weights of 3kDa, 5kDa, 10kDa, 30kDa, and 50kDa. kDa, 50.4kDa and 67.3kDa low molecular weight chitosan.
[0051] 2. Preparation of disulfide bonded alkylated chitosan and determination of its physical and chemical properties
[0052] Precisely weigh 260 mg of the cross-linking agent 3,3'-dithiodipropionic acid in the prescribed amount, dissolve it in an appropriate amount of dimethyl sulfoxide, add stearylamine (stearylamine) at a molar ratio of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| surface potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com