Conjugate, targeted tumor active oxygen responsive medicine carrying nano-micelle as well as preparation methods and application thereof
A drug-loaded nano-conjugate technology, applied in the field of medicine, can solve the problems of lack of specific recognition ability of tumor tissue, difficulty in achieving therapeutic effect, and limited penetration ability, and achieve good biocompatibility, low cost, and improved targeted effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The present invention is described in detail by the following examples, they are only used to further illustrate the present invention, can not be interpreted as the limitation of protection scope of the present invention, those skilled in the art make some non-essential improvements and improvements according to the above-mentioned content of the present invention The adjustments all belong to the protection scope of the present invention.
[0050] Example 1:
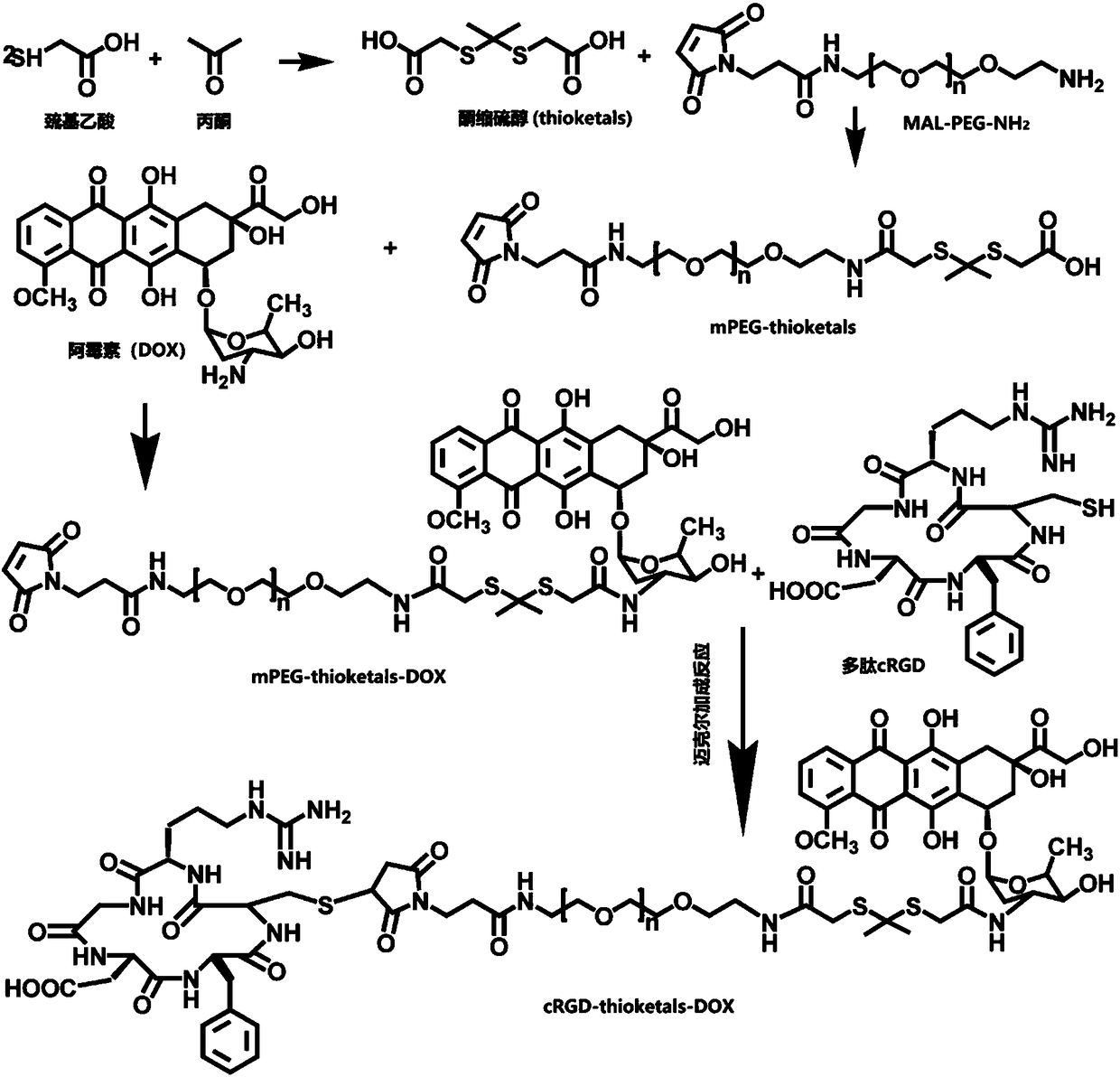
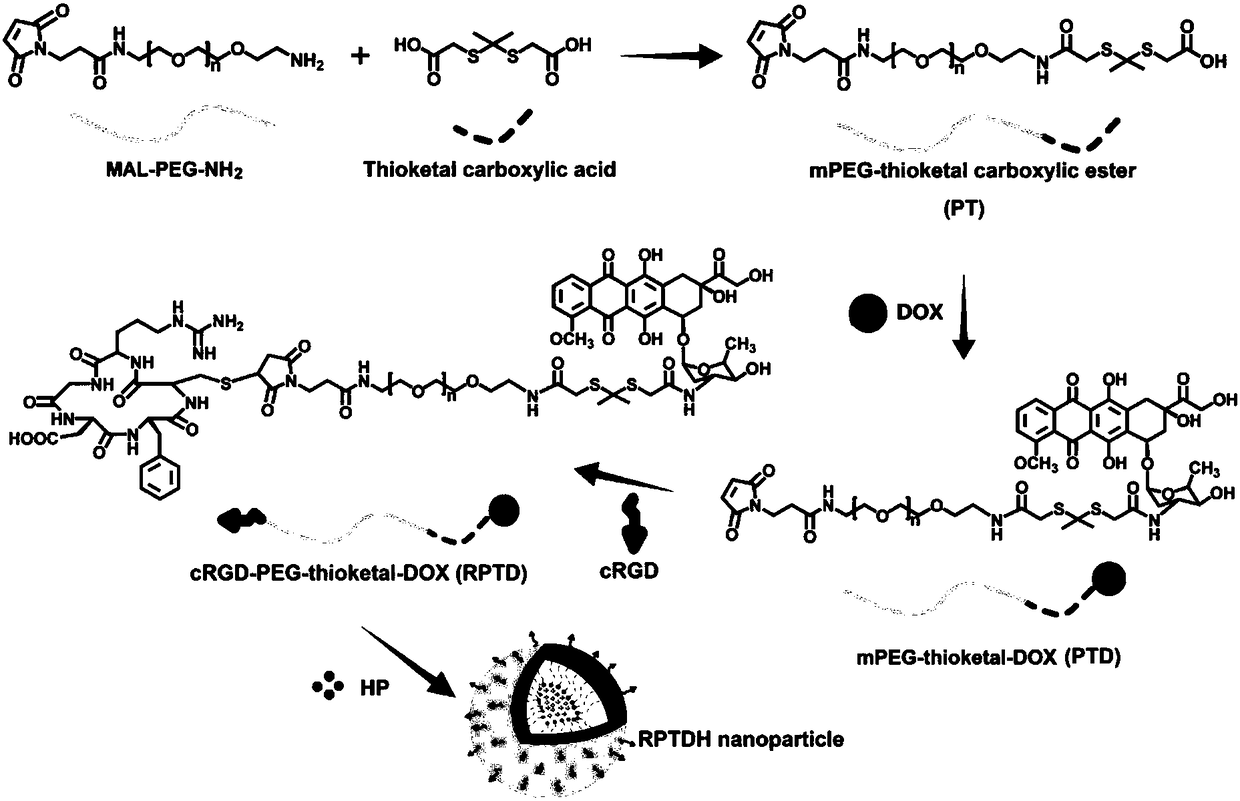
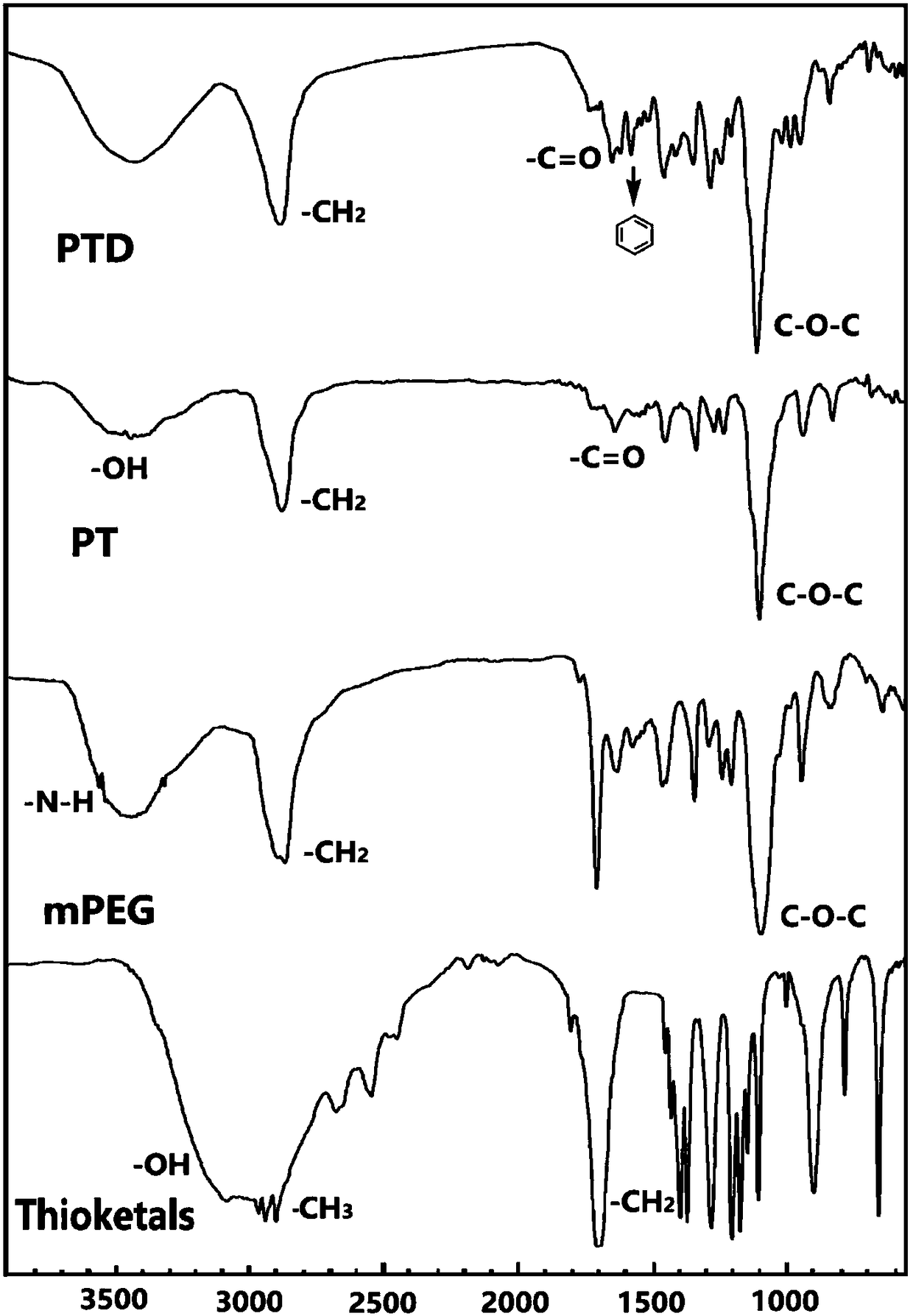
[0051] 1. Synthesis and characterization of PTD
[0052] ① Dissolve carboxylated thioketal and mPEG in DMSO at a concentration of 5 mg / ml and 10 mg / ml, respectively, and then mix them at a molar ratio of 2:1, and rotate at 500 rpm for 36 hours under strict light-proof conditions. Subsequently, the reaction solution was transferred to a dialysis bag (molecular weight cut-off 500), and dialyzed in distilled water for 36 hours, and the water was changed once every 12 hours to remove DMSO and unreacted carboxylated...
Embodiment 2
[0087] 1. Preparation of mPEG-Thioketals-epirubicin (EPB) PTE
[0088] ① Dissolve carboxylated thioketal and mPEG in DMSO at concentrations of 5 mg / ml and 10 mg / ml, respectively, and then mix them at a molar ratio of 2:1, and rotate at 500 rpm for 36 hours under strict light-proof conditions. Subsequently, the reaction solution was transferred to a dialysis bag (molecular weight cut-off 500), and dialyzed in distilled water for 36 hours, and the water was changed every 12 hours to remove DMSO and unreacted carboxylated thioketal, and a white powder was obtained after freeze-drying The product mPEG-ketonethiol (PT).
[0089] ② Dissolve epirubicin (EPB) in DMSO at a concentration of 5 mg / ml, add three times the equivalent of triethylamine, and stir at room temperature for 12 hours at a speed of 500 rpm in the dark for desalination.
[0090]③Add the above desalted EPB into PT dissolved in DMSO at 5mg / ml, the molar ratio of EPB to PT is 1:1.5, and rotate at 500rpm for 36h under s...
Embodiment 3
[0096] 1. Preparation of mPEG-Thioketals-daunorubicin (DNR) PTD
[0097] ① Dissolve carboxylated thioketal and mPEG in DMSO at a concentration of 5 mg / ml and 10 mg / ml, respectively, and then mix them at a molar ratio of 2:1, and rotate at 500 rpm for 36 hours under strict light-proof conditions. Subsequently, the reaction solution was transferred to a dialysis bag (molecular weight cut-off 500), and dialyzed in distilled water for 36 hours, and the water was changed once every 12 hours to remove DMSO and unreacted carboxylated thioketal, and a white powder was obtained after freeze-drying The product mPEG-ketonethiol (PT).
[0098] ② Dissolve daunorubicin (DNR) in DMSO at a concentration of 5 mg / ml, add three times the equivalent of triethylamine, and stir at room temperature for 12 hours at a speed of 500 rpm in the dark for desalination.
[0099] ③Add the above desalted DNR into PT dissolved in DMSO at 5mg / ml, the molar ratio of DNR to PT is 1:1.5, and rotate at 500rpm for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com