Candesartan cilexetil double-release capsule and preparation method thereof
A technology of candesartan medoxomil and double-release capsules is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., and can solve the problem of poor maintenance of blood drug concentration, decreased blood pressure, and slow dissolution rate. and other problems, to achieve the effect of high maintenance of blood drug concentration, effective antihypertensive effect and fast onset time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
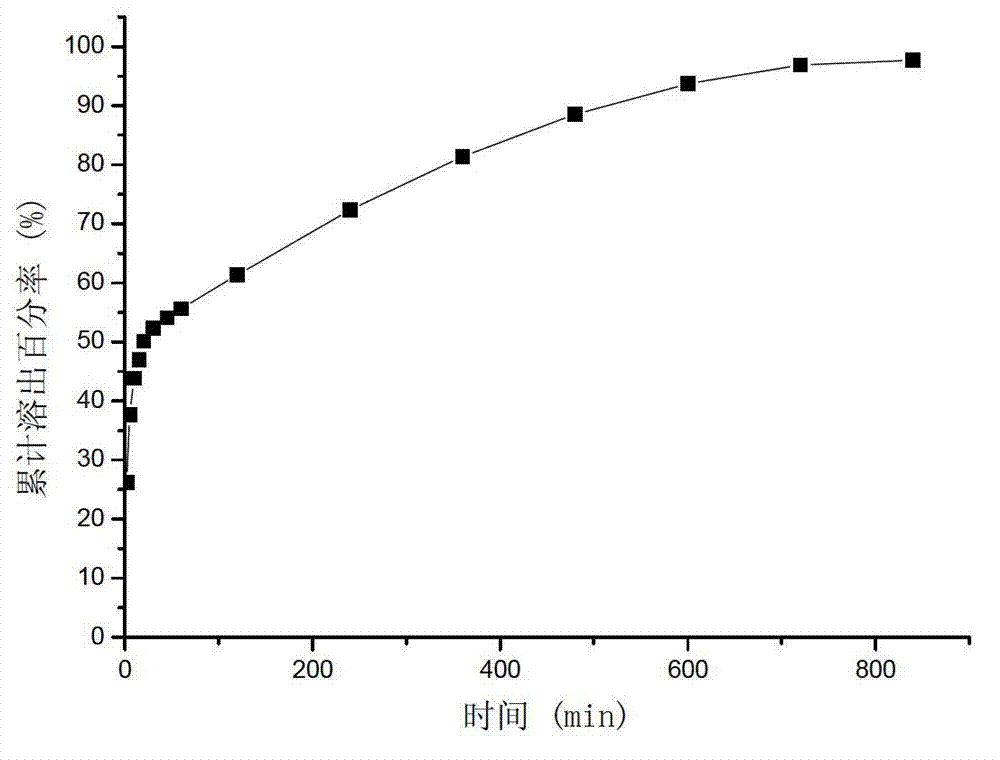
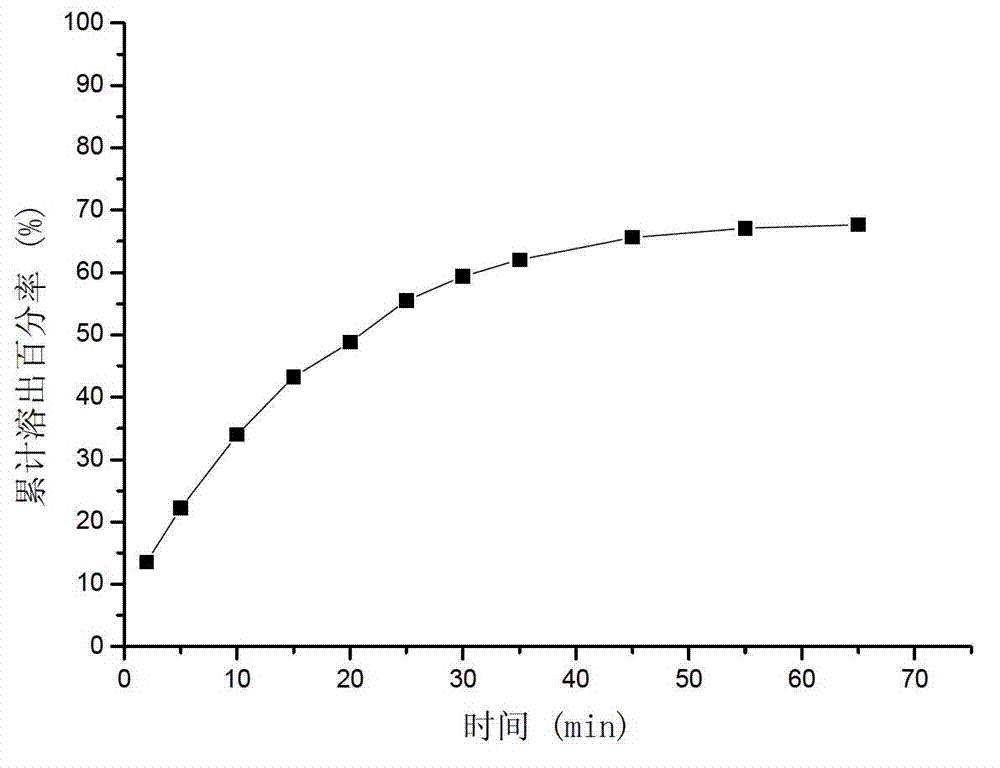
Image
Examples
Embodiment 1
[0066] Preparation of immediate-release candesartan cilexetil solid dispersion
[0067] prescription:
[0068] Candesartan cilexetil: 6g
[0069] PEG6000: 30g
[0070] Poloxamer 188: 6g
[0071] Absolute ethanol: 90mL
[0072] Acetone: 15mL
[0073] The quick-release candesartan cilexetil solid dispersion is specifically prepared as follows:
[0074] Add 30g of PEG6000 and 6g of poloxamer 188 weighed into the reaction flask, then add 75mL of absolute ethanol, heat to 35°C and stir until dissolved to obtain the first solution;
[0075] Add the weighed 6g of candesartan cilexetil into another reaction flask, then add a mixed solvent of 15mL of absolute ethanol and 15mL of acetone, stir at room temperature until dissolved and clear, and obtain the second solution;
[0076] Slowly add the second solution obtained above into the first solution, stir for 15 minutes to make the mixture uniform, heat up to 45°C and carry out vacuum distillation to remove the solvent in the syste...
Embodiment 2
[0096] Preparation of immediate-release candesartan cilexetil solid dispersion
[0097] prescription:
[0098] Candesartan cilexetil: 6g
[0099] PEG6000: 24g
[0100] Poloxamer 188: 12g
[0101] Absolute ethanol: 90mL
[0102] Acetone: 15mL
[0103] The quick-release candesartan cilexetil solid dispersion is specifically prepared as follows:
[0104] Add 24g of PEG6000 and 12g of poloxamer 188 weighed into the reaction flask, then add 75mL of absolute ethanol, heat to 35°C and stir until dissolved to obtain the first solution;
[0105] Add the weighed 6g of candesartan cilexetil into another reaction flask, then add a mixed solvent of 15mL of absolute ethanol and 15mL of acetone, stir at room temperature until dissolved and clear, and obtain the second solution;
[0106] Slowly add the second solution obtained above to the first solution, stir for 15 minutes to make the mixture uniform, heat up to 55°C and carry out vacuum distillation to remove the solvent in the syste...
Embodiment 3
[0125] Preparation of immediate-release candesartan cilexetil solid dispersion
[0126] prescription:
[0127] Candesartan cilexetil: 3g
[0128] PEG6000: 15g
[0129] Poloxamer 188: 3g
[0130] Absolute ethanol: 45mL
[0131] Acetone: 5mL
[0132] The concrete of quick-release candesartan cilexetil solid dispersion is prepared according to the following method:
[0133] Add 15g of PEG6000 and 3g of poloxamer 188 weighed into the reaction flask, then add 40mL of absolute ethanol, heat to 35°C and stir until dissolved to obtain the first solution;
[0134] Add the weighed 3g of candesartan cilexetil into another reaction flask, then add a mixed solvent of 5mL of absolute ethanol and 5mL of acetone, stir at room temperature until dissolved and clear, and obtain the second solution;
[0135] Slowly add the second solution obtained above to the first solution, stir for 15 minutes to make the mixture uniform, and then heat up to 50°C to conduct vacuum distillation to remove t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com