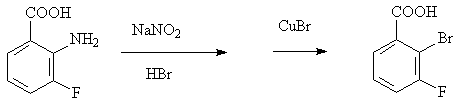
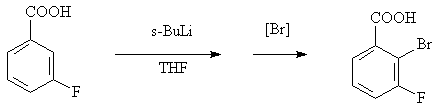
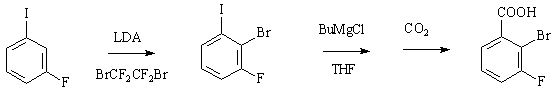
Preparation method of 2-bromine-3-fluorobenzoic acid
A technology for fluorobenzoic acid and fluorotrifluorotoluene is applied in the field of preparation of 2-bromo-3-fluorobenzoic acid, and achieves the effects of mild process conditions, reasonable process design, and cheap and readily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
Embodiment 1
[0031] Example 1, a preparation method of 2-bromo-3-fluorobenzoic acid, which uses m-fluorobenzotrifluoride as a raw material, and finally obtains the target product 2- Bromo-3-fluorobenzoic acid; the procedure is as follows:
[0032] (1) Nitration: Using sulfuric acid as a solvent, m-fluorobenzotrifluoride and nitric acid are used for nitration reaction. The molar ratio of m-fluorobenzotrifluoride to nitric acid is 1.0:1.6, and the reaction temperature is 20°C. The reaction produces 4-fluoro- 2-trifluoromethylnitrobenzene;
[0033] (2) Bromination: use sulfuric acid as solvent and dibromohydantoin as bromination reagent, wherein the molar ratio of 4-fluoro-2-trifluoromethylnitrobenzene to dibromohydantoin is 1.2:1.0, and the reaction temperature It is 20°C; the bromination reaction in this step yields 3-bromo-4-fluoro-2-trifluoromethylnitrobenzene, 5-bromo-4-fluoro-2-trifluoromethyl Brominated mixtures of nitrobenzene;
[0034] (3) Reduction: In the water phase, acetic aci...
Embodiment 2
[0038] Example 2, a preparation method of 2-bromo-3-fluorobenzoic acid, which uses m-fluorobenzotrifluoride as a raw material, and finally obtains the target product 2- Bromo-3-fluorobenzoic acid; the procedure is as follows:
[0039] (1) Nitration: Using sulfuric acid as a solvent, m-fluorobenzotrifluoride and nitric acid are used for nitration reaction. The molar ratio of m-fluorobenzotrifluoride to nitric acid is 1.0: 2.0, and the reaction temperature is 30°C. The reaction produces 4-fluoro- 2-trifluoromethylnitrobenzene;
[0040](2) Bromination: use sulfuric acid as solvent and dibromohydantoin as bromination reagent, wherein the molar ratio of 4-fluoro-2-trifluoromethylnitrobenzene to dibromohydantoin is 1.4:1.0, and the reaction temperature It is 25°C; the bromination reaction in this step yields 3-bromo-4-fluoro-2-trifluoromethylnitrobenzene, 5-bromo-4-fluoro-2-trifluoromethyl Brominated mixtures of nitrobenzene;
[0041] (3) Reduction: In the water phase, acetic aci...
Embodiment 3
[0045] Example 3, a preparation method of 2-bromo-3-fluorobenzoic acid, which uses m-fluorobenzotrifluoride as a raw material, and finally obtains the target product 2- Bromo-3-fluorobenzoic acid; the procedure is as follows:
[0046] (1) Nitration: Using sulfuric acid as a solvent, m-fluorobenzotrifluoride and nitric acid are used for nitration reaction. The molar ratio of m-fluorobenzotrifluoride to nitric acid is 1.0:1.8, and the reaction temperature is 25°C. The reaction produces 4-fluoro- 2-trifluoromethylnitrobenzene;
[0047] (2) Bromination: use sulfuric acid as solvent and dibromohydantoin as bromination reagent, wherein the molar ratio of 4-fluoro-2-trifluoromethylnitrobenzene to dibromohydantoin is 1.3:1.0, and the reaction temperature It is 22°C; the bromination reaction in this step yields 3-bromo-4-fluoro-2-trifluoromethylnitrobenzene, 5-bromo-4-fluoro-2-trifluoromethyl Brominated mixtures of nitrobenzene;
[0048] (3) Reduction: In the water phase, acetic aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com