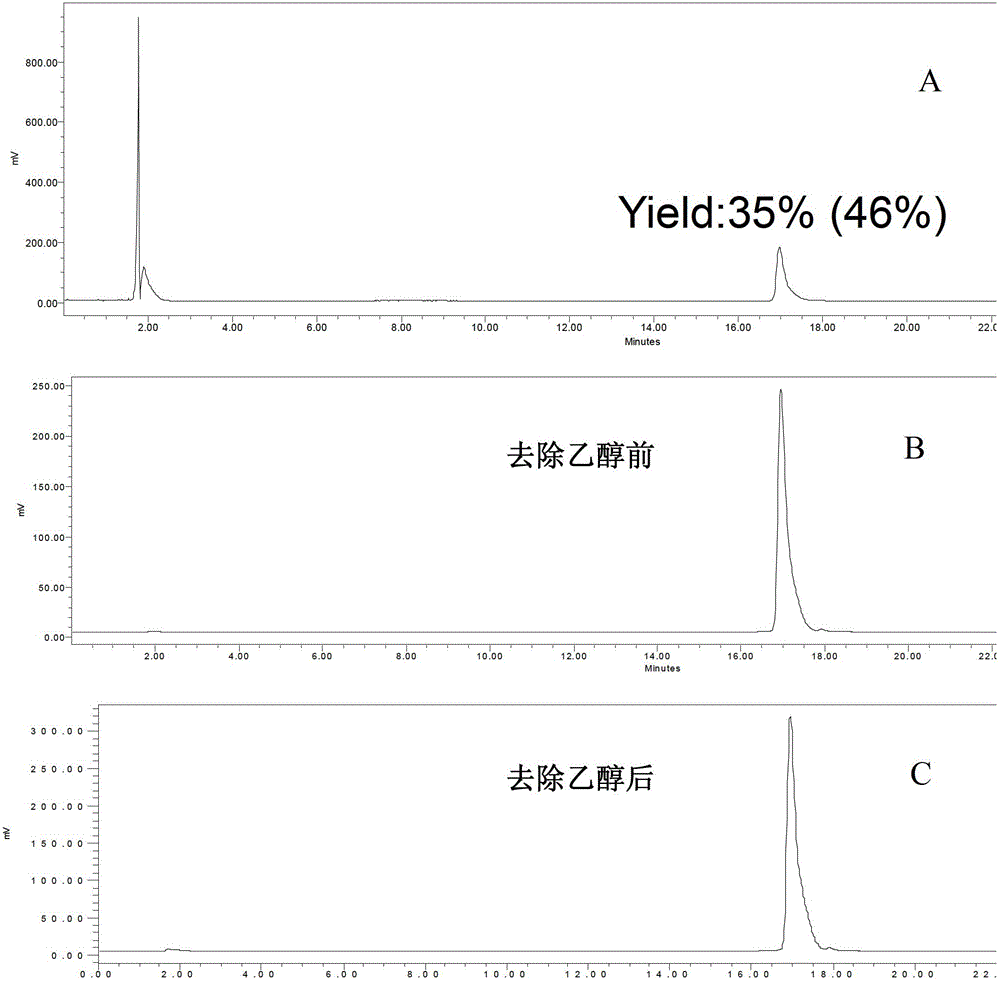
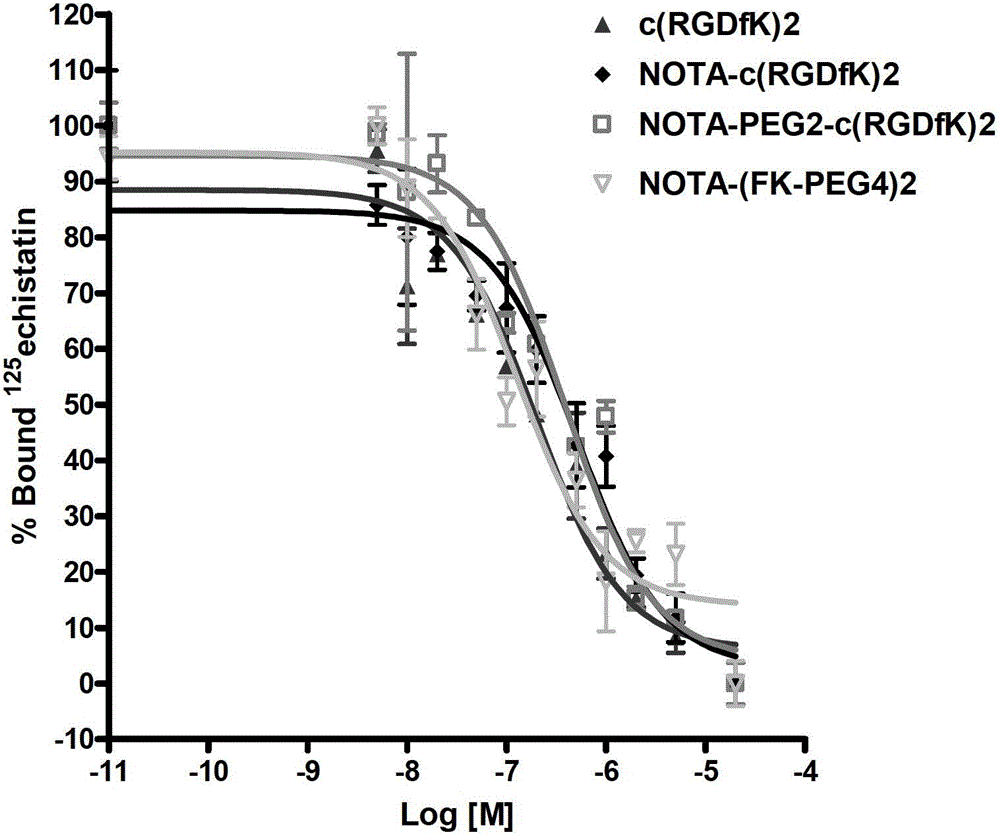
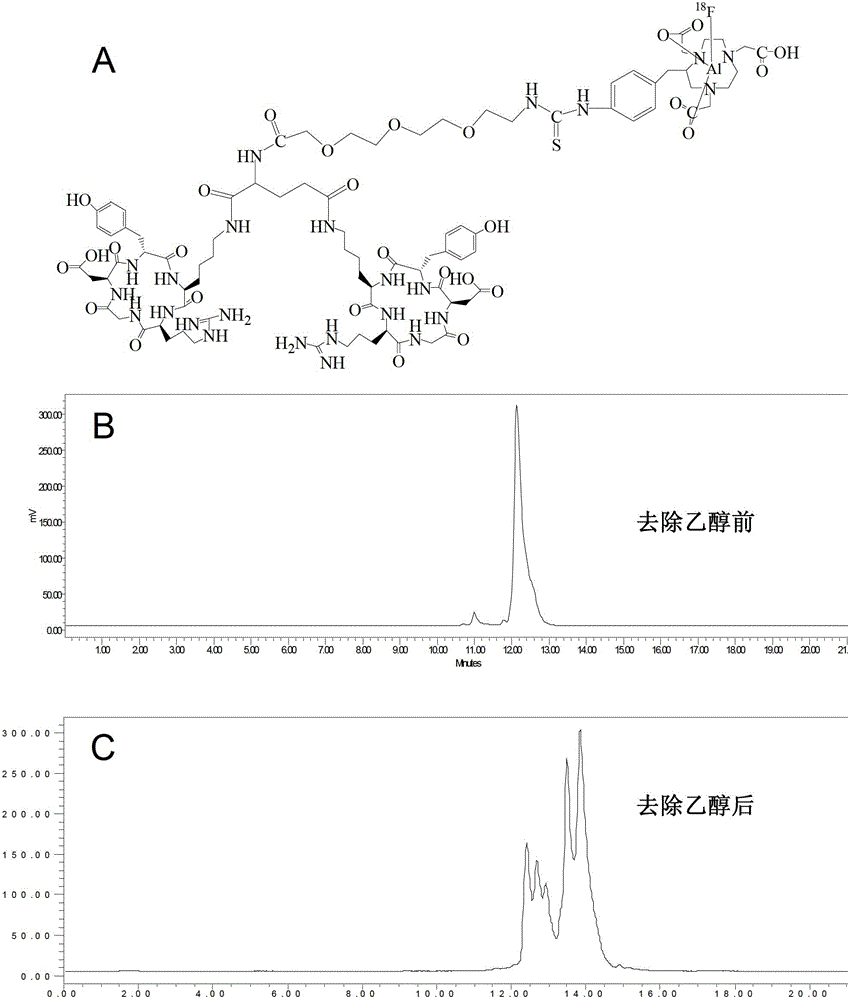
A radiolabeled polypeptide complex and its preparation method and application
A ligand compound and product technology, applied in the production of peptides and bulk chemicals, can solve the problems of inability to detect biochemical changes in tumor tissue, affect the imaging quality of tracers, poor specificity and sensitivity, and achieve easy automatic synthesis , Improve pharmacokinetic properties, increase the effect of target uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0056] 1) Preparation of PEG4-c(RGDfK)
[0057] 0.5 mmol of Boc (t-Butylcarbamate, tert-butoxycarbonyl)-protected PEG4 was dissolved in 5 mL of DMF, 0.6 mmol of NHS and 0.6 mmol of DCC were added, and reacted at room temperature for 3 hours. Add 0.4 mmolc(RGDfK) (cyclized RGD polypeptide) to the above reaction solution, adjust the pH to 8~8.5 with DIEA, and react at room temperature for 8 hours. Add 6 mL of ammonium acetate buffer (0.5 mol / L, pH=7) to the reaction solution and filter. The filtrate was separated and purified by high performance liquid chromatography (HPLC), and about 140 mg of the product Boc-PEG4-c (RGDfK) was obtained. It was confirmed as the target product by mass spectrometry (theoretical value is 1665.85).
[0058] Deprotection: add 3mL trifluoroacetic acid to 10mgBoc-PEG4-c(RGDfK), react at room temperature for 30 minutes, remove TFA by rotary evaporation, and dissolve the residue in 2mL ammonium acetate buffer (0.5mol / L, pH=7) . It was purified by HP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com