Nano ferric hydroxide and preparation method thereof
A kind of iron oxyhydroxide and nanotechnology, applied in the field of nano iron oxyhydroxide and its preparation, can solve the problems of unfavorable final product commercial application, unfavorable organic matter adsorption and decomposition, high temperature and time, etc., achieve excellent photocatalytic performance, raw material Inexpensive, agglomeration-preventing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The concrete steps of preparation are:
[0027] Step 1, first mix ethylene glycol and deionized water according to the volume ratio of 0.8:1.2, and then sonicate for 5 minutes to obtain an aqueous solution of ethylene glycol. Add ferrous sulfate in the aqueous ethylene glycol solution for ultrasonication for 5 minutes according to the weight ratio of 1.8:90 to obtain a ferrous sulfate mixture; add urea into the aqueous glycol solution for ultrasonication for 5 minutes according to the ratio of 1.3:30 for the weight ratio , to obtain the urea mixture.
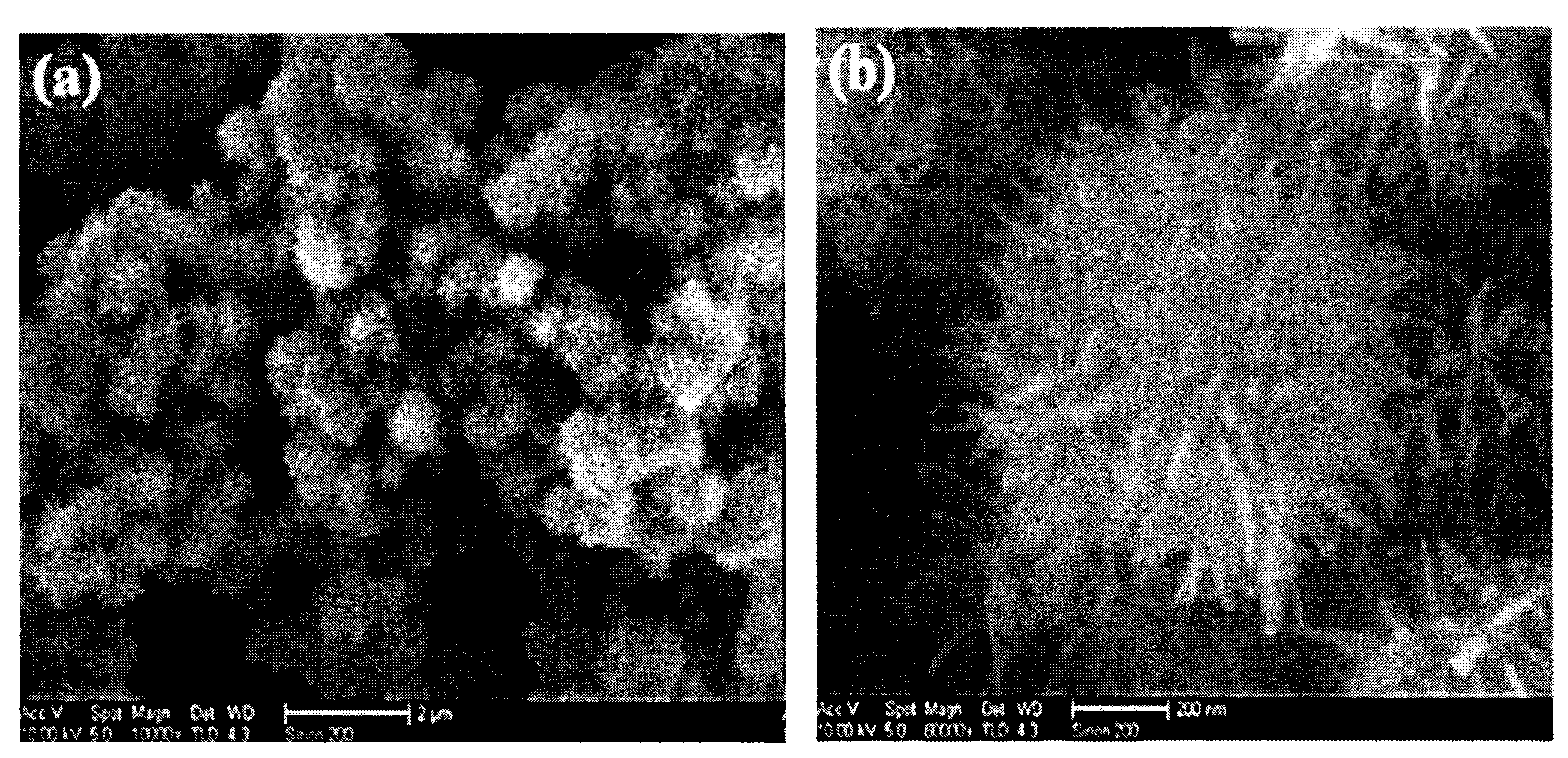
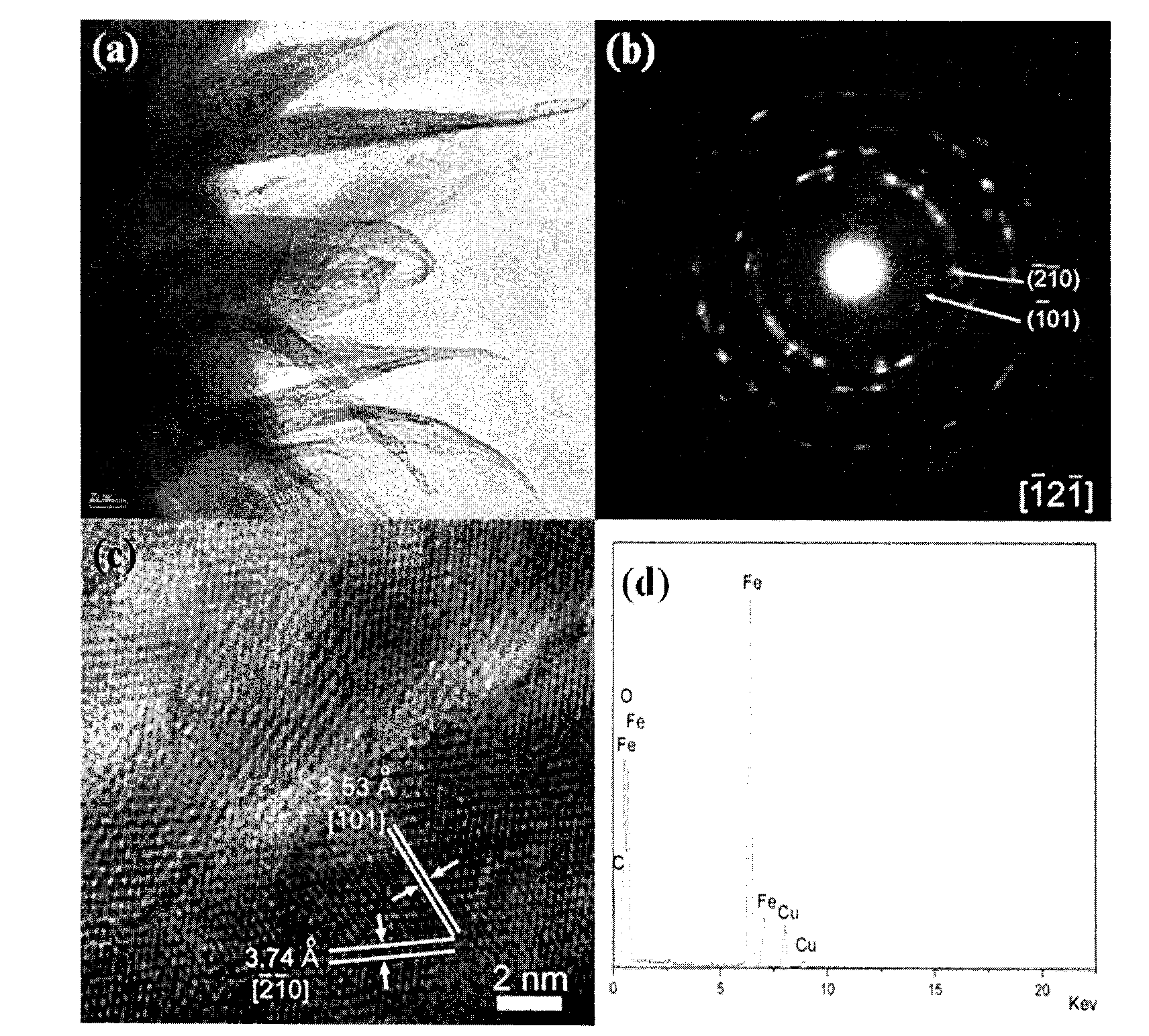
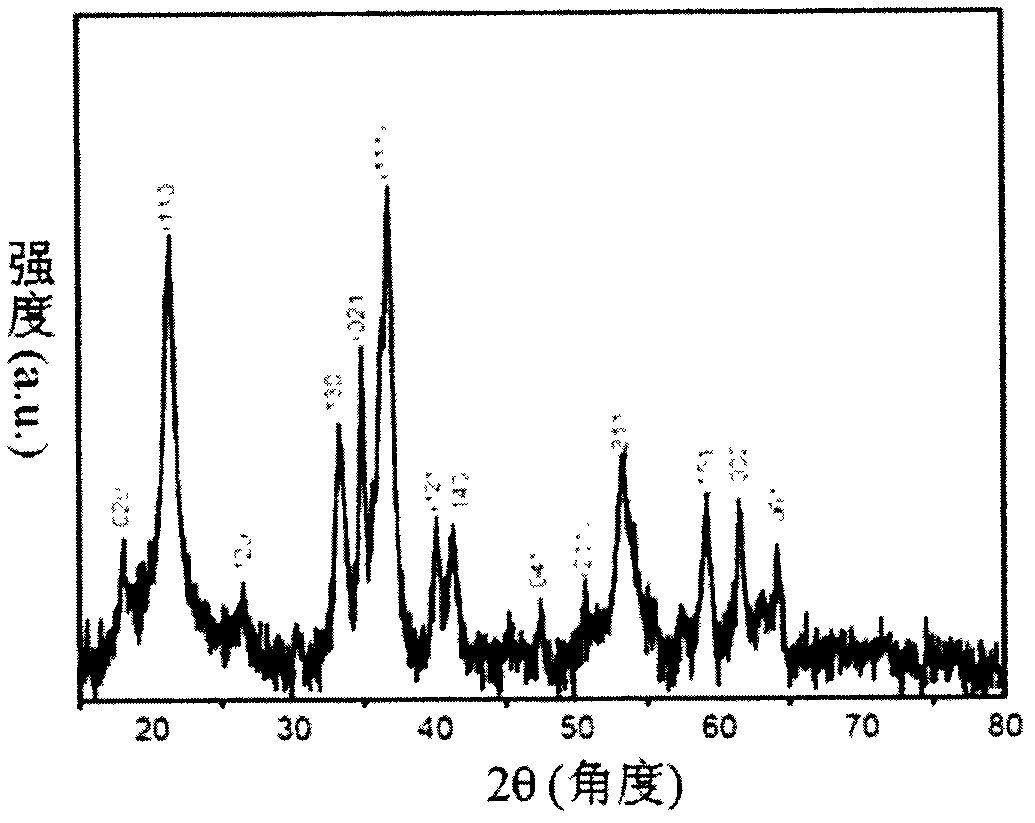
[0028] Step 2: first inject the urea mixed solution at a rate of 0.8ml / min into the stirred ferrous sulfate mixed solution at a temperature of 90° C. according to the volume ratio of 0.8:3 to obtain a reaction solution. Inject air into the reaction solution for 3h at a rate of 2.5ml / min to obtain a mixture similar to figure 1 and figure 2 shown, and as image 3 , Figure 4 and Figure 5 The nanometer iron oxyhydrox...
Embodiment 2
[0030] The concrete steps of preparation are:
[0031] Step 1, first mix ethylene glycol and deionized water according to the volume ratio of 0.9:1.1, and then sonicate for 6 minutes to obtain an aqueous solution of ethylene glycol. Add ferrous sulfate to ethylene glycol aqueous solution and ultrasonicate for 6 minutes according to the ratio of 1.9:90 by weight to obtain a ferrous sulfate mixture; add urea to ethylene glycol aqueous solution for 6 minutes according to the ratio of 1.4:30 by weight , to obtain a urea mixture.
[0032] Step 2: first inject the urea mixed solution at a rate of 0.9 ml / min into the stirred ferrous sulfate mixed solution at a temperature of 91° C. according to the volume ratio of 0.9:3 to obtain a reaction solution. Then inject air into the reaction solution for 2.8h at a rate of 2.8ml / min to obtain a mixture similar to figure 1 and figure 2 shown, and as image 3 , Figure 4 and Figure 5 The nanometer iron oxyhydroxide shown in the curve. ...
Embodiment 3
[0034] The concrete steps of preparation are:
[0035] Step 1, first mix ethylene glycol and deionized water according to the volume ratio of 1:1, and then sonicate for 7 minutes to obtain an aqueous solution of ethylene glycol. Then add ferrous sulfate into the aqueous ethylene glycol solution and ultrasonicate for 7 minutes according to the ratio of 2:90 by weight to obtain a ferrous sulfate mixture; add urea into the aqueous glycol solution and ultrasonicate for 7 minutes according to the ratio of 1.5:30 by weight , to obtain the urea mixture.
[0036] Step 2: first inject the urea mixed solution into the stirred ferrous sulfate mixed solution at a temperature of 93° C. at a rate of 1 ml / min according to a volume ratio of 1:3 to obtain a reaction solution. Inject air at a rate of 3ml / min for 2.5h into the reaction solution to obtain the following figure 1 and figure 2 shown, and as image 3 , Figure 4 and Figure 5 The nanometer iron oxyhydroxide shown in the curve....
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com