Bromhexine hydrochloride crystal as well as preparation method and application of crystal
A kind of technology of bromhexine hydrochloride and crystal, applied in the field of bromhexine hydrochloride crystal and its preparation and use, can solve problems such as unfavorable impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 : the preparation method of bromhexine hydrochloride
[0063]
[0064] (1) Synthesis of 2-amino-3,5-dibromobenzyl alcohol
[0065] Suspend 11.2g (0.04mol) of 2-amino-3,5-dibromobenzaldehyde in 20ml of ethanol, below 25°C (cooled in an ice-water bath), add 0.95g (0.025mol) of sodium borohydride solid in batches within 15min After the addition, stir at room temperature for 1.5h, add 50ml of distilled water to dilute, adjust the pH value to 6 with 6% hydrochloric acid at room temperature, and stir vigorously for 0.5h. Filter, wash with distilled water (20ml×3), suck dry, and dry (2h at 70°C) to obtain off-white solid 2-amino-3,5-dibromobenzyl alcohol.
[0066] (2) Synthesis of 2,4-dibromo-6-chloromethylaniline
[0067] Cooled in an ice-water bath (5-25 degrees), within 10 minutes, add 30ml SOCl 2 10 g (0.0357 mol) of 2-amino-3,5-dibromobenzyl alcohol was added in batches to the mixture, and after the addition was complete, it was stirred overnight at room...
Embodiment 2
[0073] Example 2 : prepare bromhexine hydrochloride crystallization according to disclosed technical scheme (DE2411848A1, example 3)
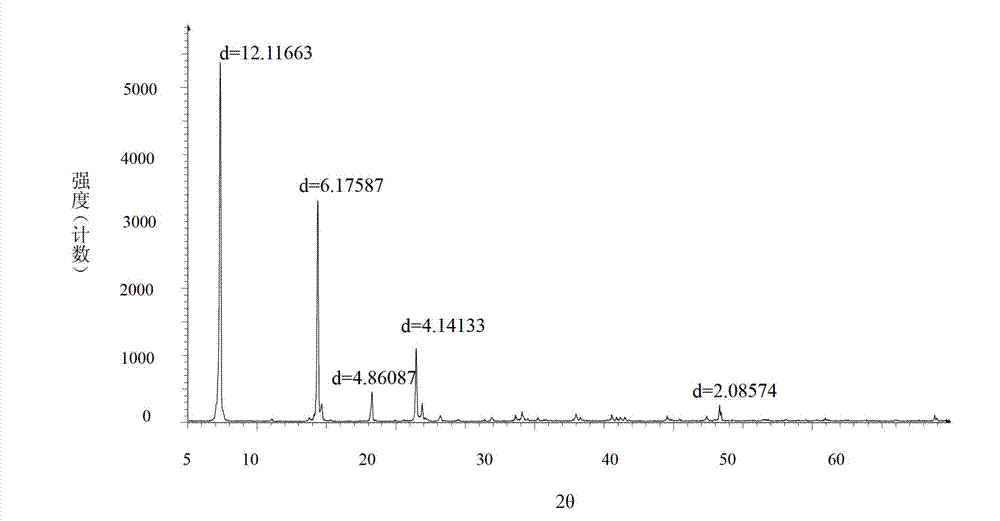
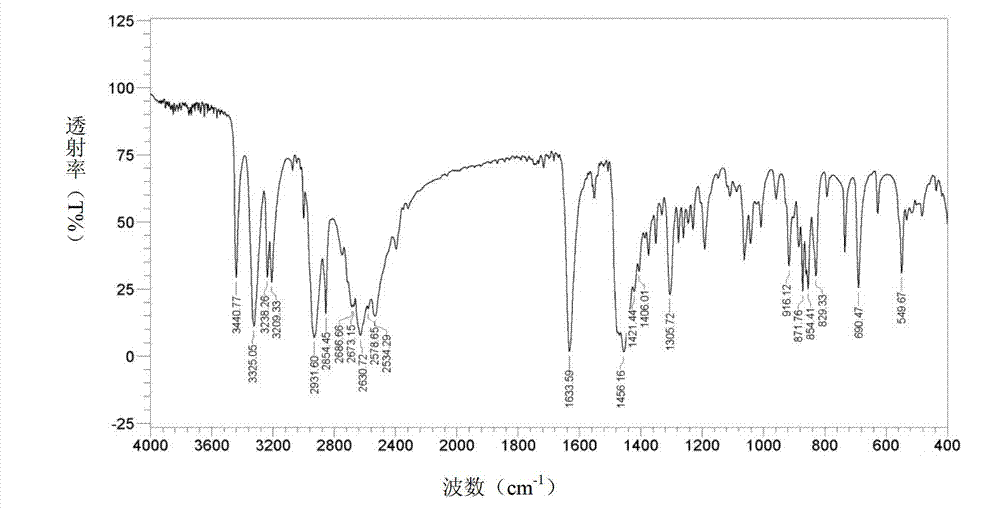
[0074] Operated by Example 1, the ethyl acetate solution of bromhexine base obtained in Example 1 was concentrated to dryness under reduced pressure, added 40ml of 17% HCl ethanol solution and 2ml of distilled water, stirred at 70°C for 1.5h, stopped heating, Add 25 ml of ethanol and stir to cool and crystallize, and filter to obtain 10 g of bromhexine hydrochloride crystals, batch number: 110101-a. Through X-diffraction and infrared measurement, the X-diffraction pattern of bromhexine hydrochloride crystal 110101-a and image 3 Consistent with the infrared absorption spectrum and Figure 4 Consistent, thus judging that the crystal form prepared by this method is consistent with crystal form I.
Embodiment 3
[0075] Example 3 : prepare bromhexine hydrochloride crystallization according to disclosed technical scheme (DE2411848A1, example 1)
[0076] According to the operation of Example 1, the ethyl acetate solution of bromhexine base obtained in Example 1 was concentrated to dryness under reduced pressure, the residue was dissolved in 80ml of absolute ethanol, and 6ml of concentrated hydrochloric acid was slowly added dropwise, and there were solids in the dropwise addition process Precipitation, dropwise, filter. The filter cake was recrystallized with 330 ml of absolute ethanol to obtain 8.9 g of bromhexine hydrochloride crystals. Batch number: 110101-b. Through X-diffraction and infrared measurement, the X-diffraction pattern of bromhexine hydrochloride crystal 110101-b and image 3 Consistent with the infrared absorption spectrum and Figure 4 Consistent, thus judging that the crystal form prepared by this method is consistent with crystal form I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com