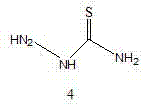
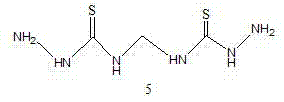
Synthesis method of bismerthiazol
A synthetic method, the technology of thiacumazole, which is applied in the field of synthesis of thiacumazole, can solve the problems of difficult post-processing, low purity and yield of thiacumazole, unfavorable environmental protection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
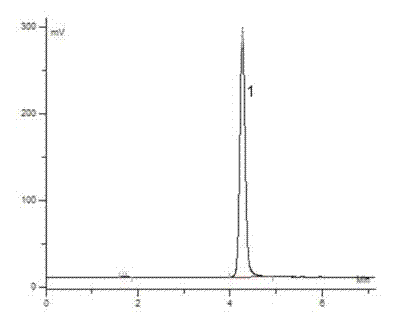
Image
Examples
Embodiment 1
[0035] Step 1: Add 125 grams of hydrazine hydrate (2mol) to a four-necked flask, add 100 grams of sulfuric acid (1.02mol) dropwise at 0°C, react at 0°C for 3 hours, add 154 grams of ammonium thiocyanate (2mol) and 10 1 g of acetone (0.17 mol) mixed solution was reacted at 70°C for 3 hours, cooled to 0°C, crystallized, and filtered to obtain 176.5 g of thiosemicarbazide (1.84 mol), with a purity of 95% and a yield of 92%.
[0036] In the second step, put 144 grams of thiosemicarbazide (1.5 mol), 36.5 grams of hydrochloric acid (1 mol), and 1 gram of dimethylformamide (0.015 mol) into the reaction bottle, and drop 23 grams of polymer Formaldehyde (0.75mol) was added dropwise, heated to 60°C, reacted for 3 hours, cooled and crystallized and filtered to obtain 138 g of thiosemicarbazone (0.75mol), with a purity of 95% and a yield of 90%.
[0037] Step 3: Put 102 grams of thiosemicarbazone (0.5 mol) and 75 grams of toluene into the reaction bottle, and add 77 grams of carbon disulf...
Embodiment 2
[0039] Step 1: Add 125 grams of hydrazine hydrate (2mol) to a four-neck flask, add 200 grams of sulfuric acid (2.04mol) dropwise at 0°C, react at 20°C for 1.5 hours, add 154 grams of ammonium thiocyanate (2mol) and 38 gram of butanone (0.54mol) mixed solution was reacted at 90°C for 2 hours, cooled to 0°C, crystallized and filtered to obtain 172.7g of thiosemicarbazide (1.77mol) with a purity of 95% and a yield of 90%.
[0040] In the second step, put 144 grams of thiosemicarbazide (1.5 mol), 36.5 grams of hydrochloric acid (1 mol), 1 gram of DMSO (0.013 mol) into the reaction bottle, and drop 50 grams of formaldehyde (1.63 mol) at 40 ° C for 2 hours. After the dropwise addition was completed, the temperature was raised to 70°C, and the reaction was carried out for 3 hours, cooled, crystallized and filtered to obtain 140.3 g of thiosemicarbazone (0.76 mol), with a purity of 92% and a yield of 91.5%.
[0041] Step 3: Put 102 grams of thiosemicarbazone (0.5mol) and 75 grams of t...
Embodiment 3
[0043] Step 1: Add 125 grams of hydrazine hydrate (2mol) to a four-neck flask, add 390 grams of sulfuric acid (4mol) dropwise at 10°C, react at 10°C for 3 hours, add 154 grams of ammonium thiocyanate (2mol) and 10 grams Acetone (0.17mol) mixed solution was reacted at 50°C for 3 hours, cooled to 0°C, crystallized, and filtered to obtain 177.5 g of thiosemicarbazide (1.85mol), with a purity of 95% and a yield of 92.5%.
[0044] In the second step, put 144 grams of thiosemicarbazide (1.5mol), 365 grams of hydrochloric acid (2mol), and 1 gram of pyridine (0.013mol) into the reaction bottle, and add 120 grams of paraformaldehyde (4mol) dropwise at 50°C for 3 hours After the dropwise addition, the temperature was raised to 80°C, and the reaction was carried out for 3 hours, cooled, crystallized and filtered to obtain 141 g of thiosemicarbazone (0.77 mol), with a purity of 94.8% and a yield of 92%.
[0045]The third step: put 102 grams of thiosemicarbazone (0.5 mol) and 75 grams of c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com