Polypyrrole/ferrite/multi-wall carbon nanotube composite material preparation method
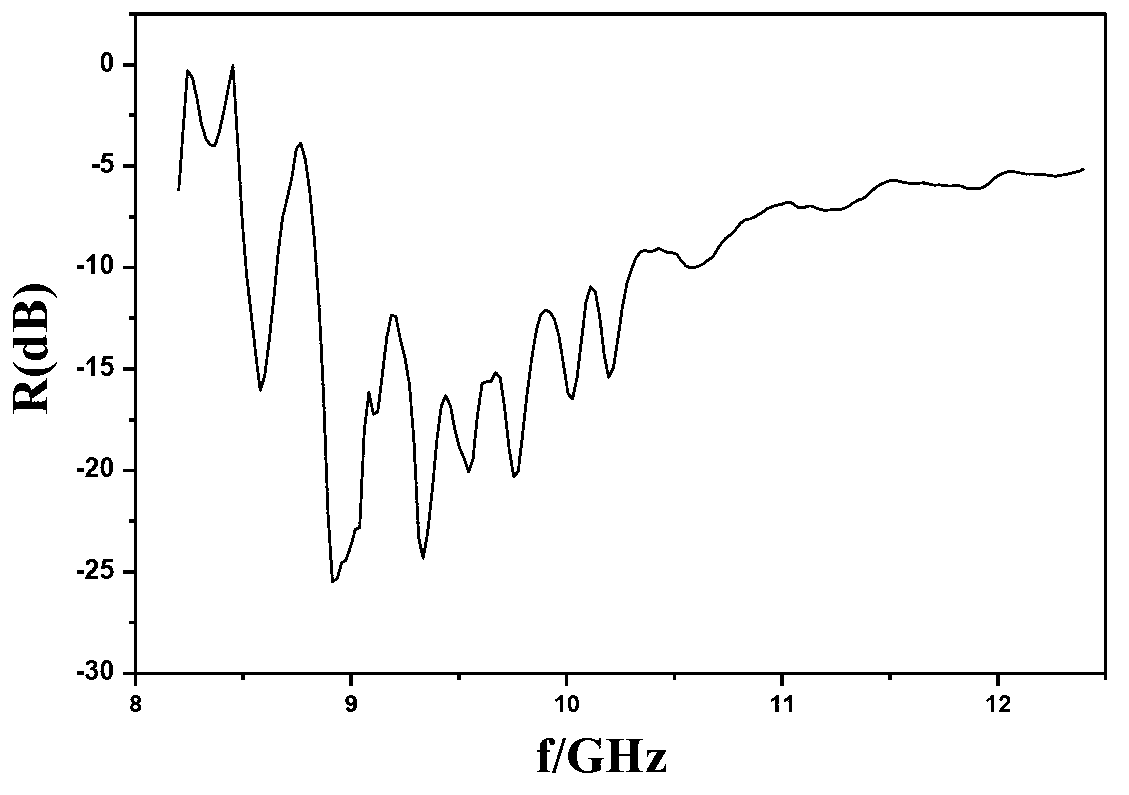
A technology of multi-wall carbon nanotubes and composite materials, which is applied in the field of preparation of polypyrrole/ferrite/multi-wall carbon nanotube composite materials, and can solve problems such as poor wave-absorbing performance, small wave-absorbing loss, and low electrical conductivity. problem, to achieve the effect of broadening the microwave absorption frequency band, good microwave absorption performance, and good conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
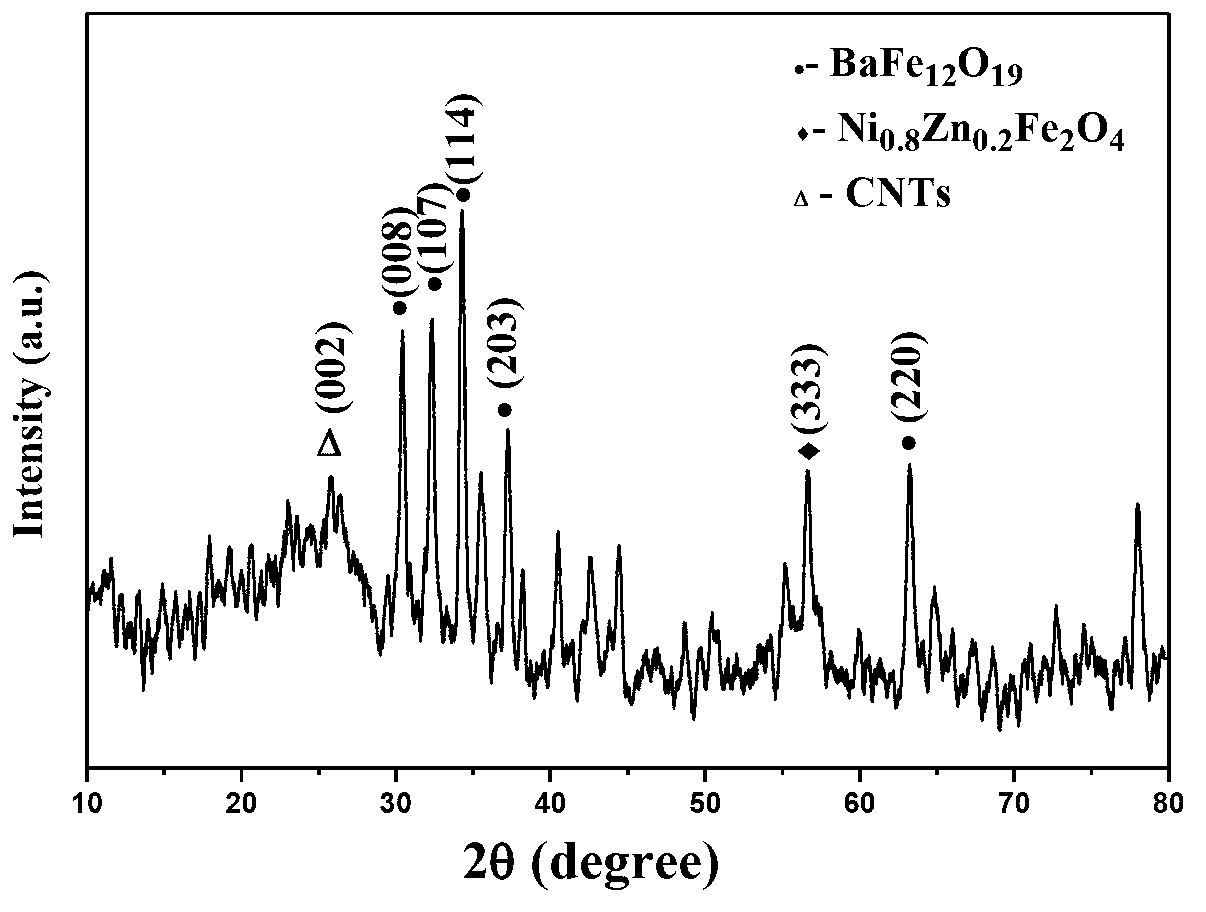
[0019] (1) BaFe 12 o 19 -Ni 0.8 Zn 0.2 Fe 2 o 4 Preparation of:
[0020] (a) Dissolve citric acid, iron nitrate, nickel nitrate, zinc nitrate, and barium nitrate with a molar ratio of 217:76:4:1:6 in deionized water, and magnetically stir evenly at room temperature;
[0021] (b) ethylene glycol with a molar ratio of 2 times citric acid is added to the solution, and then the pH is adjusted to 7 with ethylenediamine;
[0022](c) under magnetic stirring, the obtained solution is heated to 75° C., and the water is evaporated until a gel is formed;
[0023] (d) The gel was first dried in an oven at 120 °C for 24 h, then transferred to a muffle furnace at 400 °C for pre-sintering for 3 h, and finally calcined at 1100 °C for 2 h to obtain BaFe 12 o 19 -Ni 0.8 Zn 0.2 Fe 2 o 4 Ferrite powder.
[0024] (2) Treatment of multi-walled carbon nanotubes:
[0025] (a) first calcining the multi-walled carbon nanotubes in a tube furnace at 600°C for 2h;
[0026] (b) Transfer to a...
Embodiment 2
[0035] (1) BaFe 12 o 19 -Ni 0.8 Zn 0.2 Fe 2 o 4 Preparation of:
[0036] (a) Dissolve citric acid, iron nitrate, nickel nitrate, zinc nitrate, and barium nitrate with a molar ratio of 217:76:4:1:6 in deionized water, and magnetically stir evenly at room temperature;
[0037] (b) ethylene glycol with a molar ratio of 2 times citric acid is added to the solution, and then the pH is adjusted to 7 with ethylenediamine;
[0038] (c) under magnetic stirring, the obtained solution is heated to 75° C., and the water is evaporated until a gel is formed;
[0039] (d) The gel was first dried in an oven at 120 °C for 24 h, then transferred to a muffle furnace at 400 °C for pre-sintering for 3 h, and finally calcined at 1100 °C for 2 h to obtain BaFe 12 o 19 -Ni 0.8 Zn 0.2 Fe 2 o 4 Ferrite powder.
[0040] (2) Treatment of multi-walled carbon nanotubes:
[0041] (a) first calcining the multi-walled carbon nanotubes in a tube furnace at 600°C for 2h;
[0042] (b) Transfer to ...
Embodiment 3
[0051] (1) BaFe 12 o 19 -Ni 0.8 Zn 0.2 Fe 2 o 4 Preparation of:
[0052] (a) Dissolve citric acid, iron nitrate, nickel nitrate, zinc nitrate, and barium nitrate with a molar ratio of 217:76:4:1:6 in deionized water, and magnetically stir evenly at room temperature;
[0053] (b) ethylene glycol with a molar ratio of 2 times citric acid is added to the solution, and then the pH is adjusted to 7 with ethylenediamine;
[0054] (c) under magnetic stirring, the obtained solution is heated to 75° C., and the water is evaporated until a gel is formed;
[0055] (d) The gel was first dried in an oven at 120 °C for 24 h, then transferred to a muffle furnace at 400 °C for pre-sintering for 3 h, and finally calcined at 1100 °C for 2 h to obtain BaFe 12 o 19 -Ni 0.8 Zn 0.2 Fe 2 o 4 Ferrite powder.
[0056] (2) Treatment of multi-walled carbon nanotubes:
[0057] (a) first calcining the multi-walled carbon nanotubes in a tube furnace at 600°C for 2h;
[0058] (b) Transfer to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com