Ruthenium complexes, preparation method thereof and application
A ruthenium complex and complex technology, applied in the field of preparation of ruthenium polypyridine complexes, can solve the problems of increased cell drug resistance and achieve the effect of solving the problem of cell drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
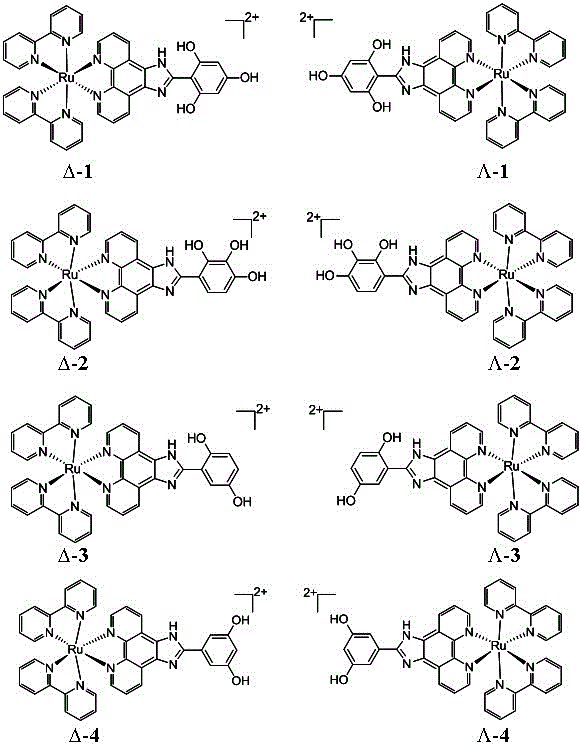
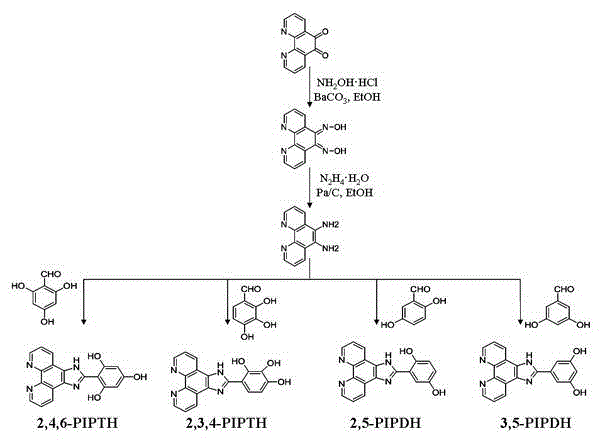
[0032] The preparation method of embodiment 1 ligand and complex
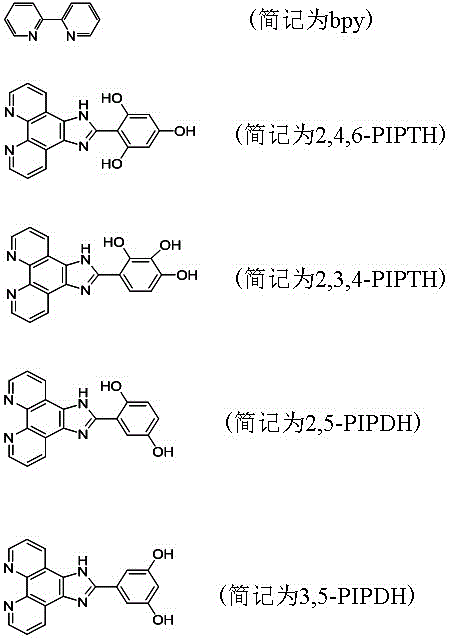
[0033] (1) Ligand 2,4,6-PIPTH; 2,3,4-PIPTH; 2,5-PIPDH; 3,5-PIPDH synthesis method:
[0034] a) The synthetic method of o-phenanthrolene-5,6-diamine:
[0035] The synthesis of o-phenanthrolene-5,6-diamine is according to ( Tetrahedron Letters , 2006, 38, 8159) Method: Dissolve 4.2g of phenanthroline-5,6-dione in 200mL of absolute ethanol, and add 4.8g of hydroxylamine hydrochloride and 5.9g of BaCO 3 , refluxed at 80°C for 12 hours, then rotary evaporated to remove the solvent, the obtained solid was dissolved with dilute HCl and the pH was adjusted to about 6, stirred for half an hour, a green solid precipitated, filtered, washed, dried and dissolved in 300mL of absolute ethanol, added 4.0 g10% palladium carbon, under the protection of Ar, start to add N dropwise after refluxing at 80°C 2 h 4 ·H 2 The mixed solution of O and ethanol (35mL / 150mL) was dropped within 1 hour and continued to pass Ar to refl...
Embodiment 2
[0055] Example 2 CD spectral analysis
[0056] In circular light, when a beam of polarized light passes through an optically active medium, there will be a phase difference between the left-handed and right-handed circular vibration components, which is the basic principle of circular dichroism. The symmetry of the chiral complexes can be obtained according to the changes in the CD spectra of the resolved isomers. The ruthenium complex of fixed concentration 10 μ M is analyzed its CD spectrum under the wavelength of 200-700nm ( Figure 4 ).
Embodiment 3
[0057] Example 3 Topoisomerase Inhibition Experiment of Ru(II) Complexes
[0058] Inhibition ability was judged according to the method of drug inhibition topoisomerase unwinding experiment. The compound was reacted with pBR322 DNA and topoisomerase in an appropriate buffer, and the reaction mixture was incubated at 37 °C for a certain period of time, and then the reaction termination solution was added to terminate the reaction. On 0.9% agar enamel (TBE) gel, electrophoresis under constant voltage conditions of 80 V. The gel was stained with 1.5 μg / mL EB solution and photographed under UV light. The complex concentration that inhibits 50% of Topo I or Topo II activity is defined as IC 50 . The experimental results show that the complex is a dual inhibitor of Topo I and Topo II, showing a very good inhibitory ability, IC 50 The value is about 3~5 mM ( Figure 5 ). And all the complexes are superior to the reported small organic molecule inhibitors in terms of water so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com