Polymer, glucose nano gel, glucose nano gel composition and preparation method thereof
A nanogel and polymer technology, applied in the field of glucose nanogel composition and its preparation, glucose nanogel, polymer, can solve the problems of poor biodegradability, application field limitation and poor biocompatibility of glucose nanogel and other issues, to achieve good biodegradability, good biocompatibility, and improve the effect of curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] The present invention also provides a method for preparing a polymer having a structure of formula (I), comprising the following steps:
[0059]reacting the polymer with the structure of formula (III) and 2-azidoethyl-D-(+)-glucopyranoside under the action of a catalyst to obtain the polymer with the structure of formula (I);
[0060]
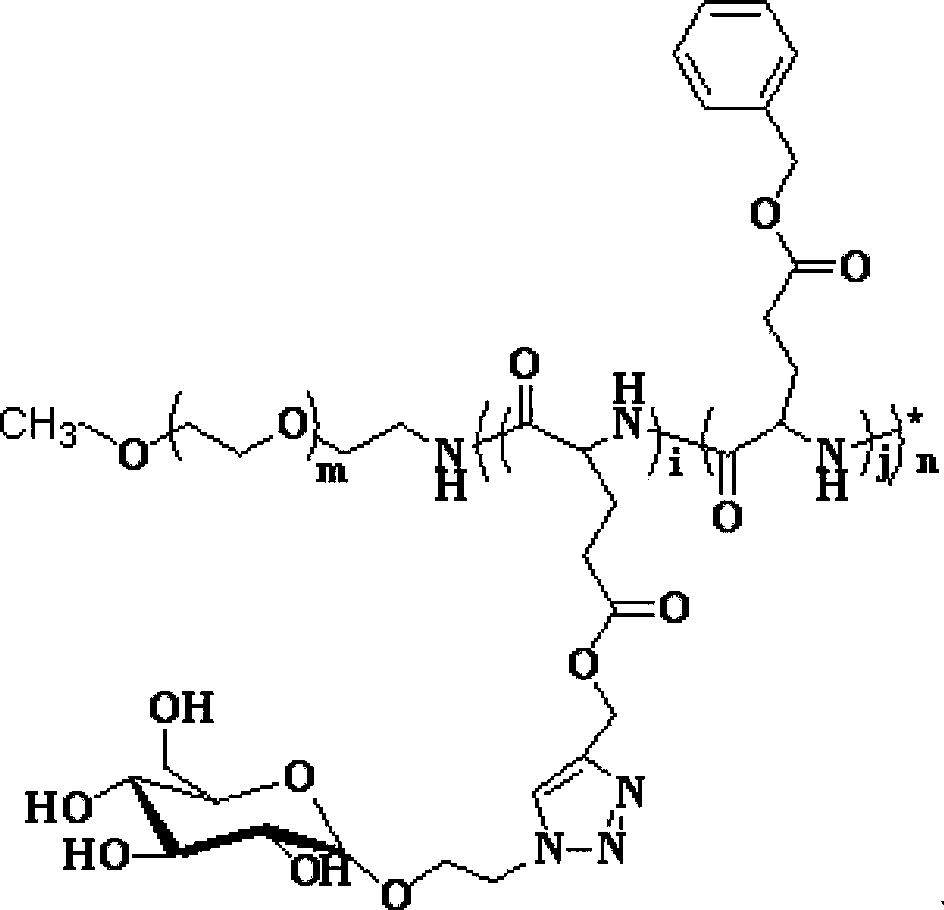
[0061] Formula (III);
[0062]
[0063] Formula (I);
[0064] Wherein, m is the degree of polymerization, 55≤m≤250, preferably 100≤m≤200, more preferably 110≤m≤150; n is the degree of polymerization, 20≤n≤150, preferably 30≤n≤120, more preferably Preferably 40≤n≤100;
[0065] 0.1≤i / (i+j)≤1, preferably 0.1≤i / (i+j)<1, more preferably 0.3≤i / (i+j)≤0.7, most preferably 0.4≤i / (i +j) ≤ 0.7.
[0066] In the present invention, the polymer having the structure of formula (III) is reacted with 2-azidoethyl-D-(+)-glucopyranoside under the action of a catalyst to obtain the polymer having the structure of formula (I) . The catalyst is pre...
Embodiment 1
[0129] After azeotroping 25g of polyethylene glycol monomethyl ether (molecular weight: 5000) with toluene to remove water, dissolve it in 150mL of anhydrous dichloromethane, add 3.5mL of triethylamine at 0°C and anhydrous conditions, and drop 8mL Methanesulfonyl chloride was reacted at 0°C for 2h, returned to 25°C, and continued to react for 48h under stirring with a stirrer. After the reaction was completed, the resulting precipitate was filtered off, and the filtrate was settled with ether, filtered, washed, and vacuumed at 25°C. After drying for 24 hours, polyethylene glycol monomethyl ether methanesulfonate was obtained.
[0130] Dissolve 3 g of the above-prepared polyethylene glycol monomethyl ether methanesulfonate and 1 g of ammonium chloride in 80 mL of ammonia water with a mass concentration of 25%, react at 25°C for 72 hours, and obtain amino-terminated polyethylene glycol after the reaction Monomethyl ether, the aminated polyethylene glycol monomethyl ether was ext...
Embodiment 2
[0132] Add 1.041 g (0.208 mmol) of amino-terminated polyethylene glycol monomethyl ether hydrochloride (mPEG-NH 2 HCl), using toluene to azeotropically remove water and then dissolving with anhydrous N,N-dimethylformamide to obtain amino-terminated polyethylene glycol monomethyl ether hydrochloride solution.
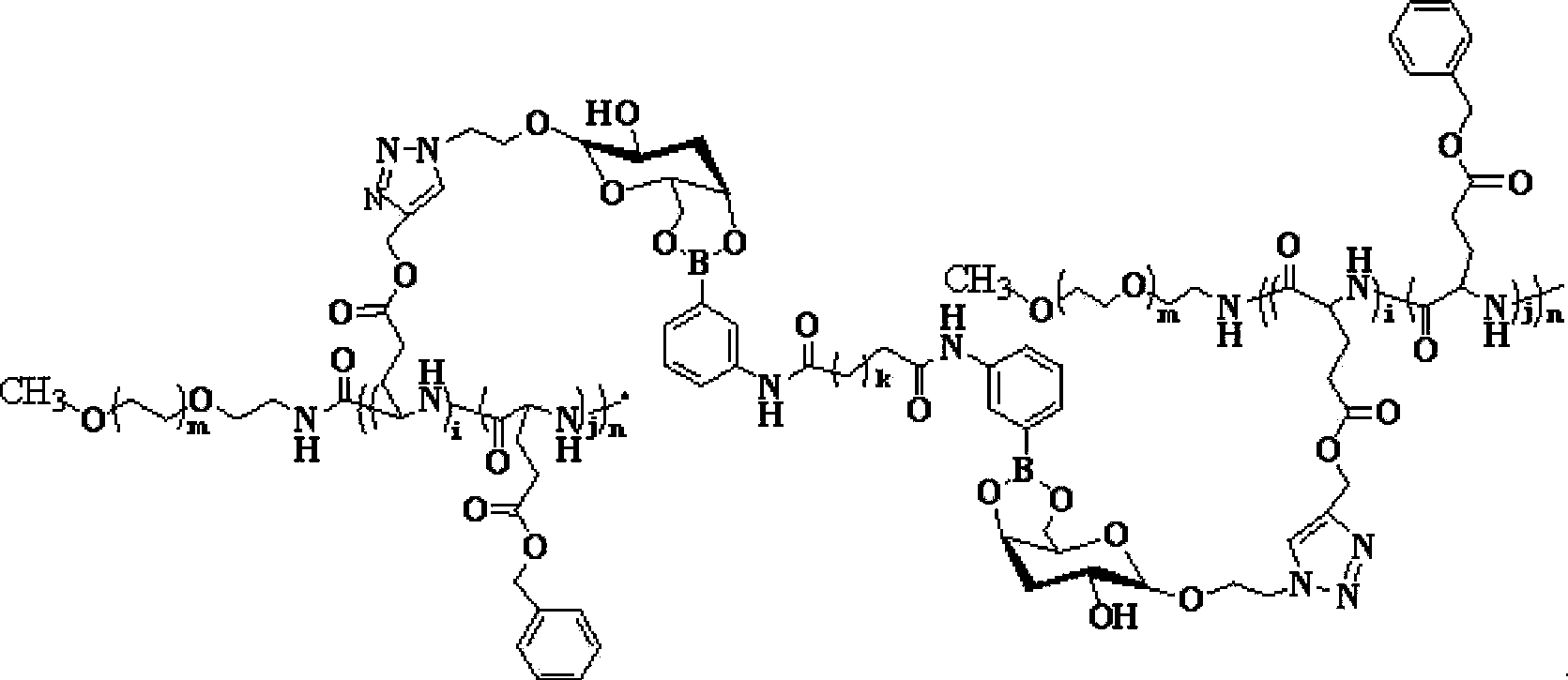
[0133]3.2875g (12.49mmol) of the compound (BLG-NCA) with the formula (IV) and 2.635g (12.49mmol) of the compound (PLG-NCA) with the formula (V) were treated with anhydrous N,N-dimethyl Formamide was dissolved and added to the amino-terminated polyethylene glycol monomethyl ether hydrochloride solution, and the reaction was carried out at 25°C under stirring with a stirrer. The reaction time was 72 hours. After the reaction was completed, the solution was poured into the volume Settled in diethyl ether 10 times the amount of the solvent, filtered, washed, and dried in vacuum at 25°C for 24 hours to obtain a polymer with the structure of formula (III) (mPEG-b-P(BLG-co-PLG)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com