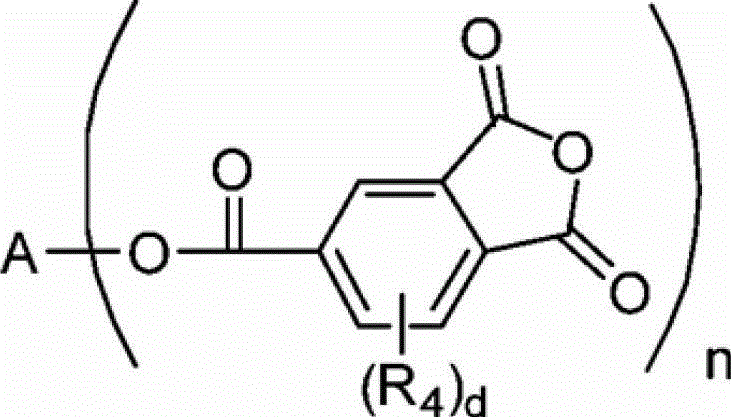
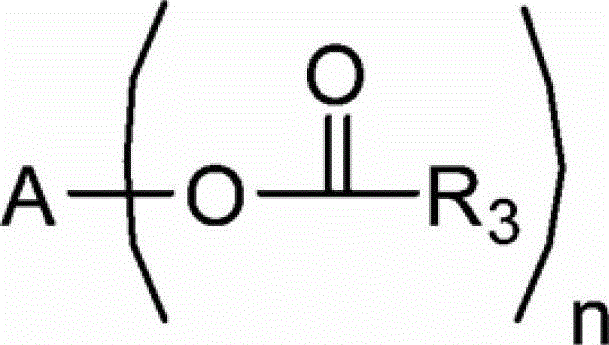
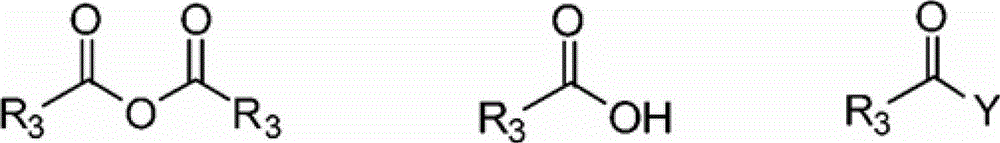
Process for producing trimellitic anhydride phenyl ester
A technology of trimellitic anhydride phenyl ester and trimellitic anhydride benzene, which is applied in the field of trimellitic anhydride phenyl esters, can solve the problems of low reaction speed, expensive catalyst, insufficient reaction selectivity, etc., and achieve increased yield, increased reaction selectivity, and selectivity. Excellent rate of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1)
[0102] Add 675.0g (6.6 moles) of acetic anhydride and 342.0g (1.5 moles) of 2,2-bis(4-hydroxyphenyl)propane into a 3L 4-neck flask equipped with a thermometer, a reflux device, and a stirring blade. After heating up to 130° C. with stirring, the reaction was further carried out at the same temperature for 2.5 hours while stirring.
[0103] After the reaction was terminated, 703 g of toluene was added and cooled, then water was added, stirred, and washed with water.
[0104] Thereafter, the water layer was separated and removed, and toluene was distilled and removed from the obtained oil layer, and 937 g of heptane was added, crystallized and filtered to obtain 2,2-bis(4 -Acetoxyphenyl) propane (hereinafter referred to as BPA-DA) crystals.
Embodiment 1)
[0106] After the 300ml four-necked flask equipped with stirrer, thermometer, Dean-Stark device, and reflux condenser was replaced with nitrogen, 25.0g (0.080 moles) of BPA-DA, 46.2g ( 0.241 mol) of trimellitic anhydride, 0.25 g (relative to BPA-DA: 4.7 mol %) of lithium acetate, and 54.5 g of diphenyl ether.
[0107] Thereafter, the temperature was raised to 210° C. under normal-pressure nitrogen flow, and the reaction was performed for 7 hours while maintaining 210° C. with stirring. The reaction was carried out while distilling off the generated acetic acid under a nitrogen stream at normal pressure. The reaction liquid after 4 hours and 7 hours after heating up to 210 degreeC was extracted, and it analyzed by GPC (gel permeation chromatography). The results are shown in Table 1.
[0108] Reaction of 2,2-bis{4-(1,3-dioxo-1,3-dihydroisobenzofuran-5-ylcarbonyloxy)phenyl}propane as target (ditrimellitic anhydride ester) The selectivity rate was 96.7%.
Embodiment 2~4)
[0114] The reaction was carried out in the same manner as in Example 1, except that the catalysts described in Table 2 were used in an amount of 4.7 mol % to BPA-DA instead of lithium acetate in Example 1. Table 2 shows the GPC analysis results of the reaction solution after the temperature was raised to 210° C. for 4 hours and 7 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com