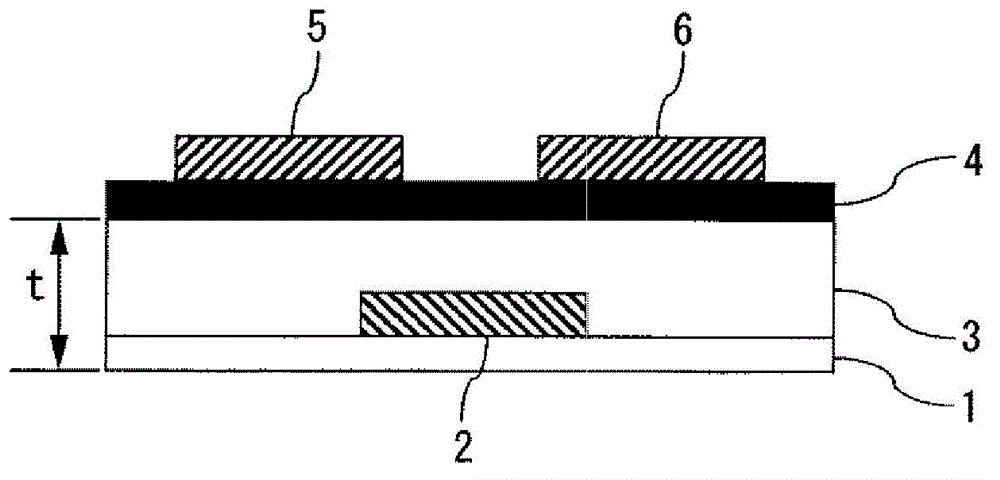
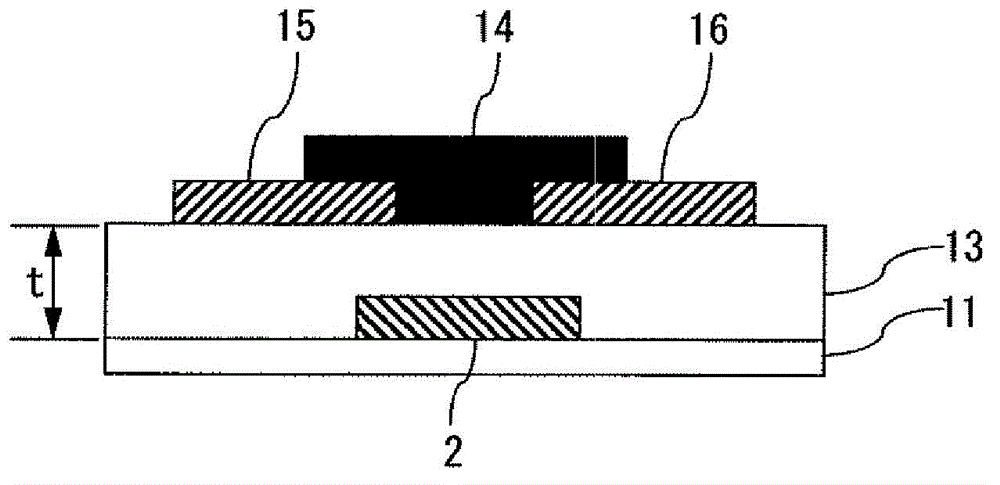
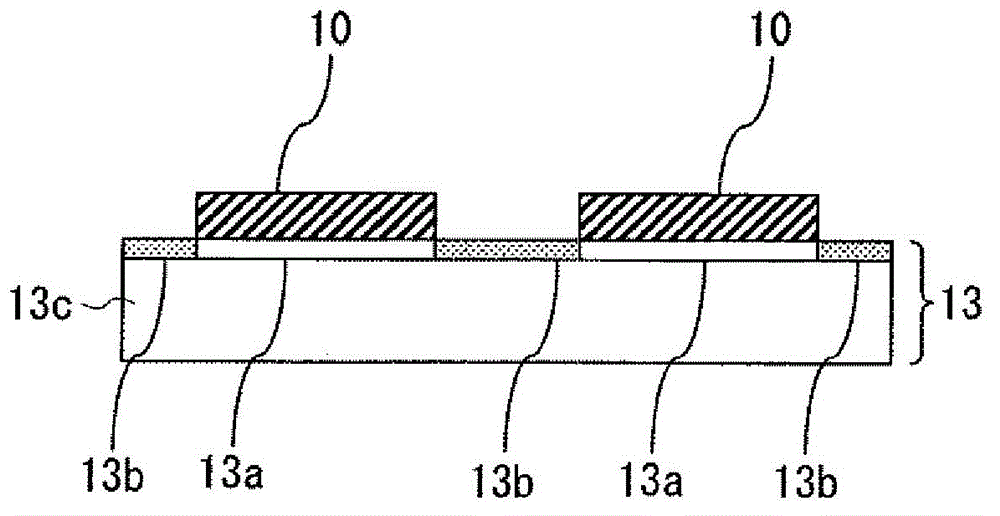
Curable composition and method for producing cured film
A curable composition and compound technology, which is applied in semiconductor/solid-state device manufacturing, electric solid-state device, coating, etc., can solve the problem that fluorine-containing polyarylene resin cannot be used, and achieve the effect of preventing warping
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0357] (Synthesis Example 1: Synthesis of Fluorinated Polyarylene Ether Prepolymer (A1))
[0358] PFB (450 g), pentafluorophenylacetylene (155 g) and 1,3,5-tri Hydroxybenzene (130g) was reacted at 60°C for 45 hours to synthesize a prepolymer (A1). The DMAc solution of the obtained prepolymer (A1) was placed in an aqueous hydrochloric acid solution (3.5% by mass aqueous solution), reprecipitated and purified, and vacuum-dried to obtain 620 g of a powdery prepolymer (A1). The number average molecular weight (Mn) of the prepolymer (A1) was 10,000.
Synthetic example 2
[0359] (Synthesis Example 2: Synthesis of Fluorinated Polyarylene Ether Prepolymer (A2))
[0360] In DMAc (492g) solvent, in the presence of pentafluorostyrene (22g), 1,1,1-tris(4-hydroxyphenyl)ethane (33g) and sodium carbonate (51g), at 60°C After making it react for 24 hours, the solution which melt|dissolved PFB (40g) in DMAc (360g) was added next, and it was made to react at 60 degreeC for 17 hours, and the prepolymer (A2) was synthesize|combined. The obtained DMAc solution of the prepolymer (A2) was placed in an aqueous hydrochloric acid solution (3.5% by mass aqueous solution), reprecipitated and purified, and vacuum-dried to obtain 750 g of a powdery prepolymer (A2). The number average molecular weight (Mn) of the prepolymer (A2) was 10,000.
Synthetic example 3
[0361] (Synthesis Example 3: Synthesis of Fluorinated Polyarylene Ether Prepolymer (A3))
[0362] In DMAc (6.2 kg) solvent, in the presence of PFB (650 g), 1,3,5-trihydroxybenzene (120 g) and potassium carbonate (570 g), after reacting at 40° C. for 6 hours, then, 4-Acetoxystyrene (200g) was made to react in presence of 48 mass % potassium hydroxide aqueous solution (530g), and the prepolymer (A3) was synthesize|combined. The obtained DMAc solution of the prepolymer (A3) was placed in an aqueous hydrochloric acid solution (3.5% by mass aqueous solution), reprecipitated and purified, and vacuum-dried to obtain 800 g of a powdery prepolymer (A3). The number average molecular weight (Mn) of the prepolymer (A3) was 10,000.
[0363]
[0364] The abbreviations indicate the following compounds.
[0365] [Monomers having a fluoroalkyl group (Cf)]
[0366] C6FMA:CH 2 =C(CH 3 ) COOCH 2 CH 2 (CF 2 ) 6 F.
[0367] C4FMA:CH 2 =C(CH 3 ) COOCH 2 CH 2 (CF 2 ) 4 F.
[0368] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com