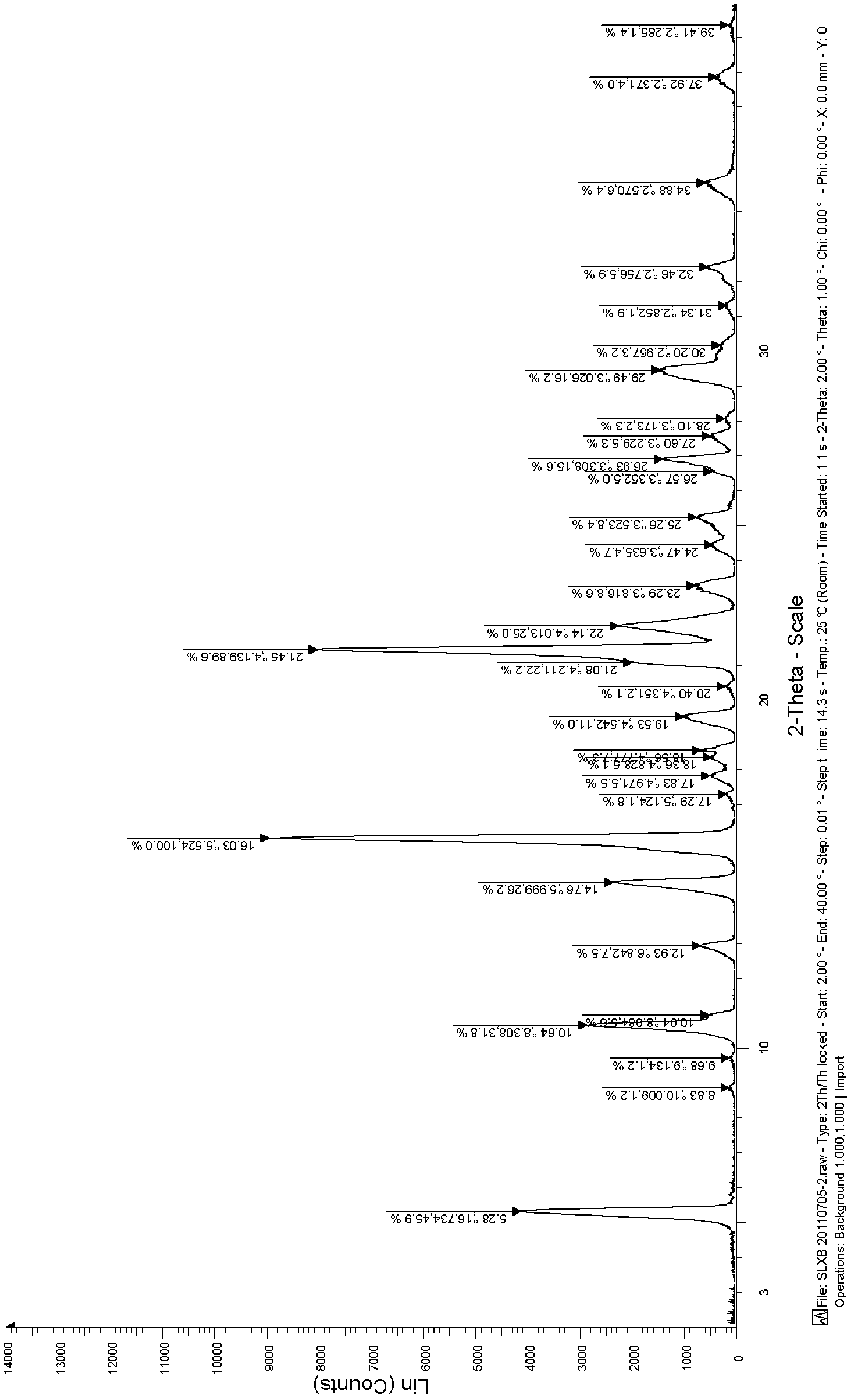
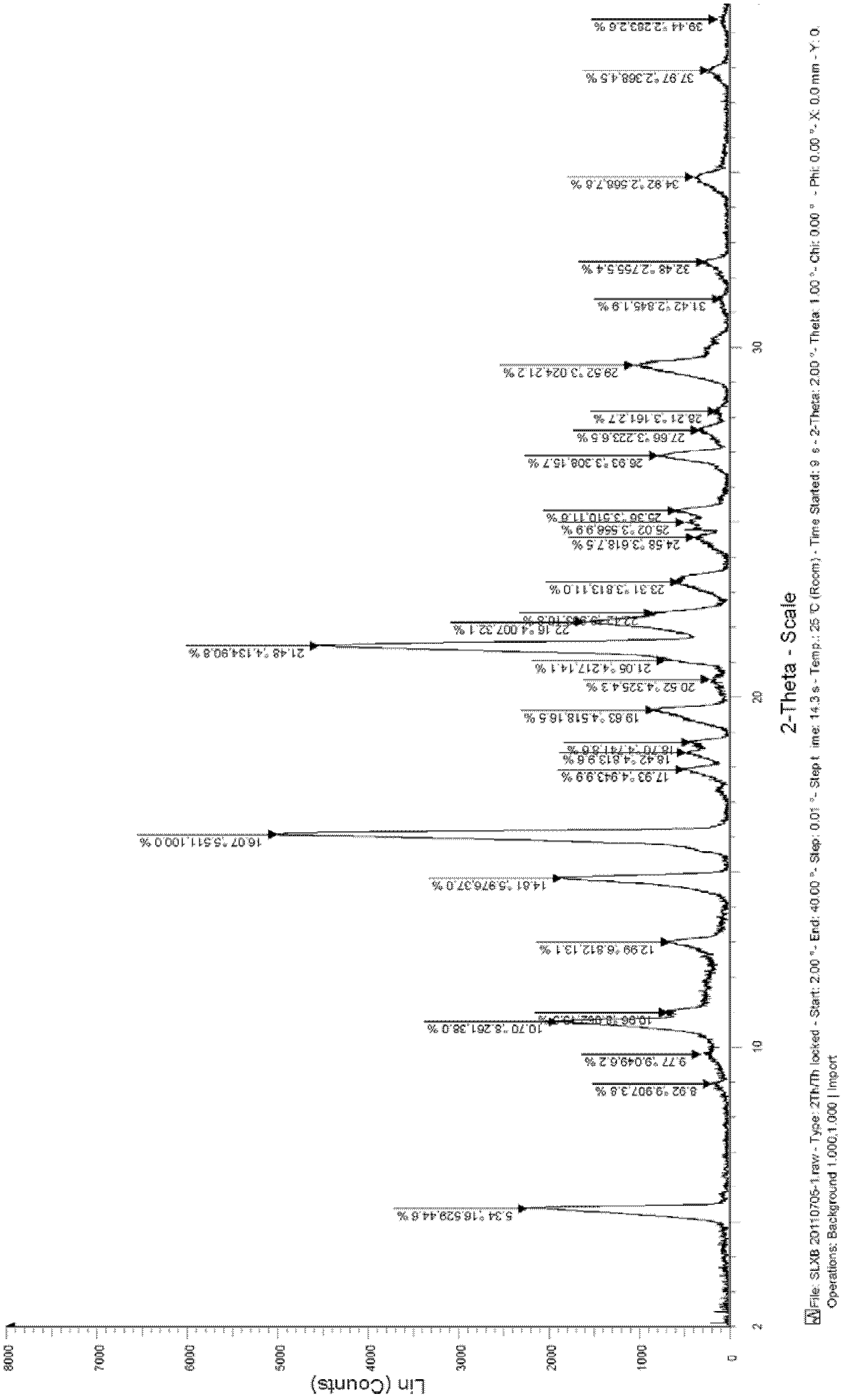
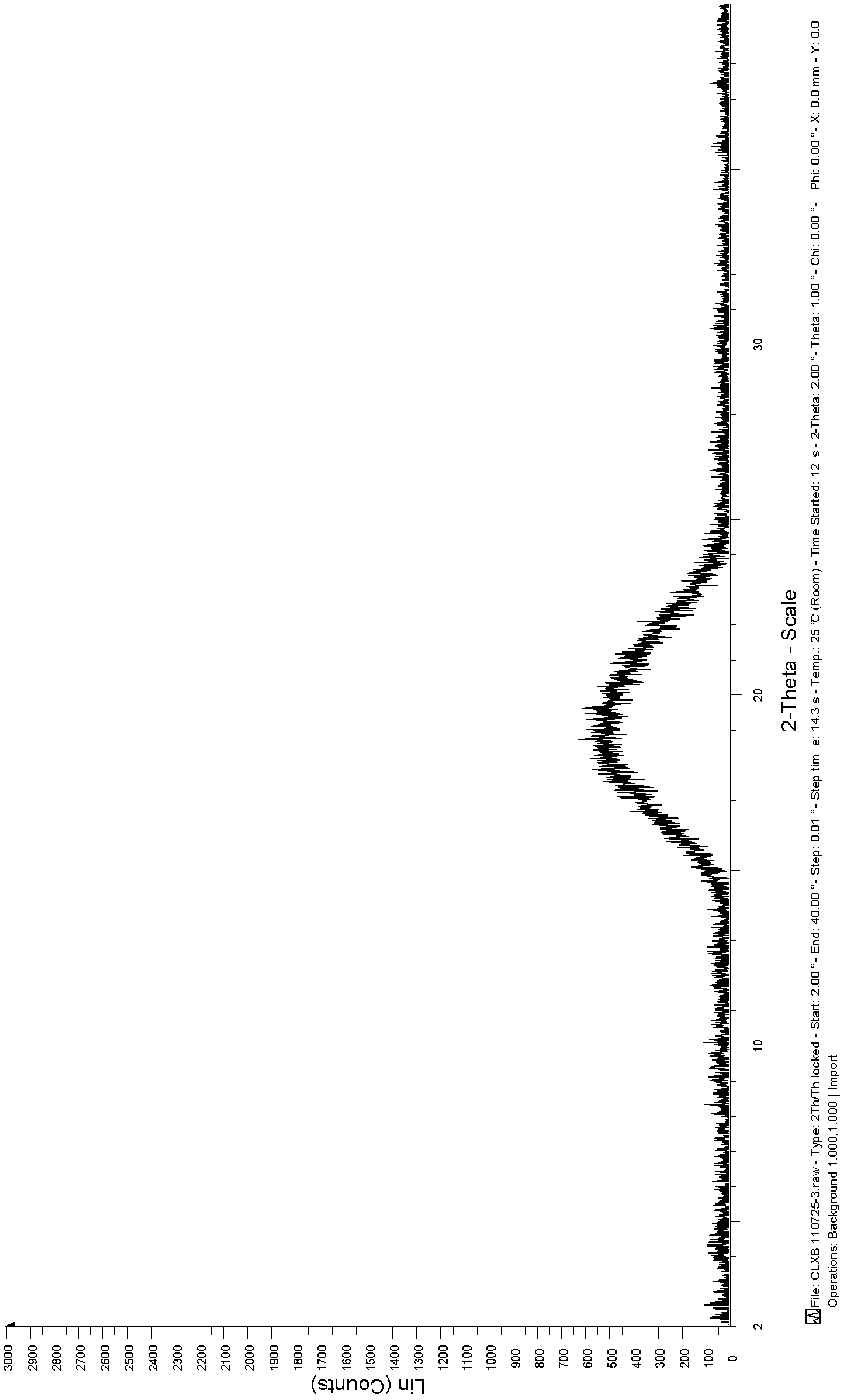
Celecoxib solid dispersion and preparation method thereof
A technology of solid dispersion and celecoxib, which is applied in the directions of medical preparations without active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, can solve the problem of slow dissolution of celecoxib raw materials and polyethylene glycol. High viscosity, difficult to dissolve evenly, etc., to achieve the effect of easy operation, high drug loading, and avoiding the crushing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] formula:
[0030]
[0031] The raw material of celecoxib is prepared according to the Chinese patent application CN1141630A.
[0032] Preparation:
[0033] The raw and auxiliary materials are weighed according to the formula amount, completely dissolved in ten times the amount of methanol, the solvent is removed by rotary evaporation, and then dried under reduced pressure in a vacuum drying oven.
Embodiment 2
[0035] formula:
[0036]
[0037] Preparation:
[0038] The raw and auxiliary materials are weighed according to the formula amount, completely dissolved in ethanol of 20 times the amount, the solvent is removed by rotary evaporation, and then dried under reduced pressure in a vacuum drying oven to obtain the product.
Embodiment 3
[0040] formula:
[0041]
[0042] Preparation:
[0043] The raw and auxiliary materials are weighed according to the formula amount, completely dissolved in five times the amount of acetone, the solvent is removed by rotary evaporation, and then dried under reduced pressure in a vacuum drying oven.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com