A kind of Qi Fumai preparation
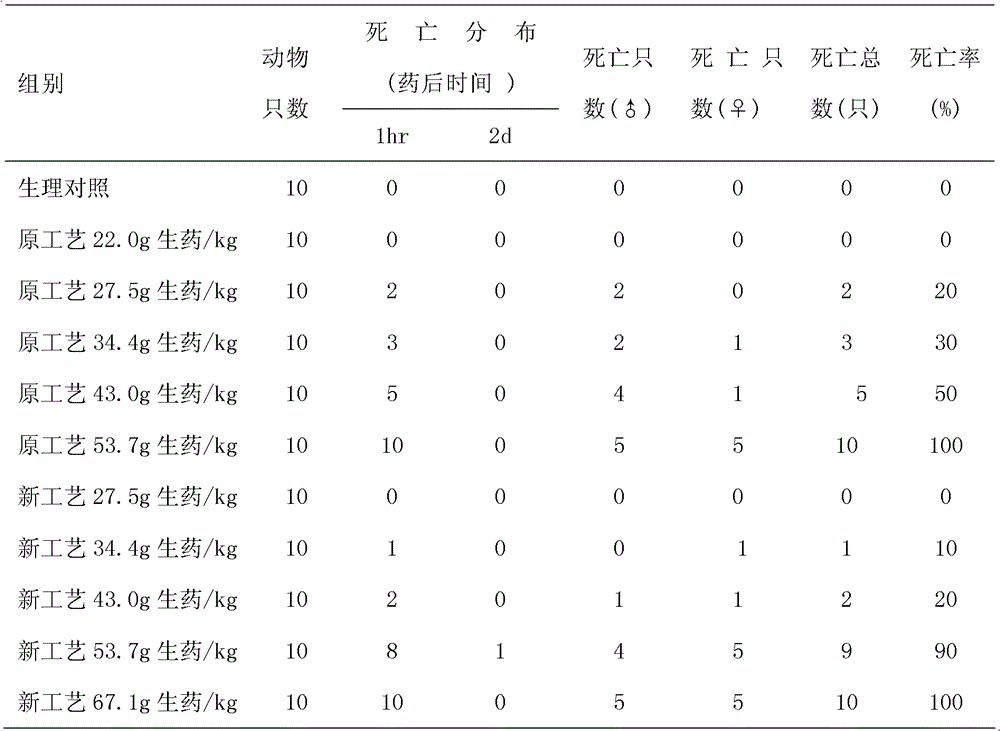
A technology for invigorating qi and regenerating pulses and preparations, which is applied in the directions of medical preparations containing active ingredients, powder transportation, freeze-drying transportation, etc. The effect of shortening the freeze-drying period and widening the range of safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 注射用益气复脉冷冻干燥制剂,
[0059] 处方如下:
[0060]
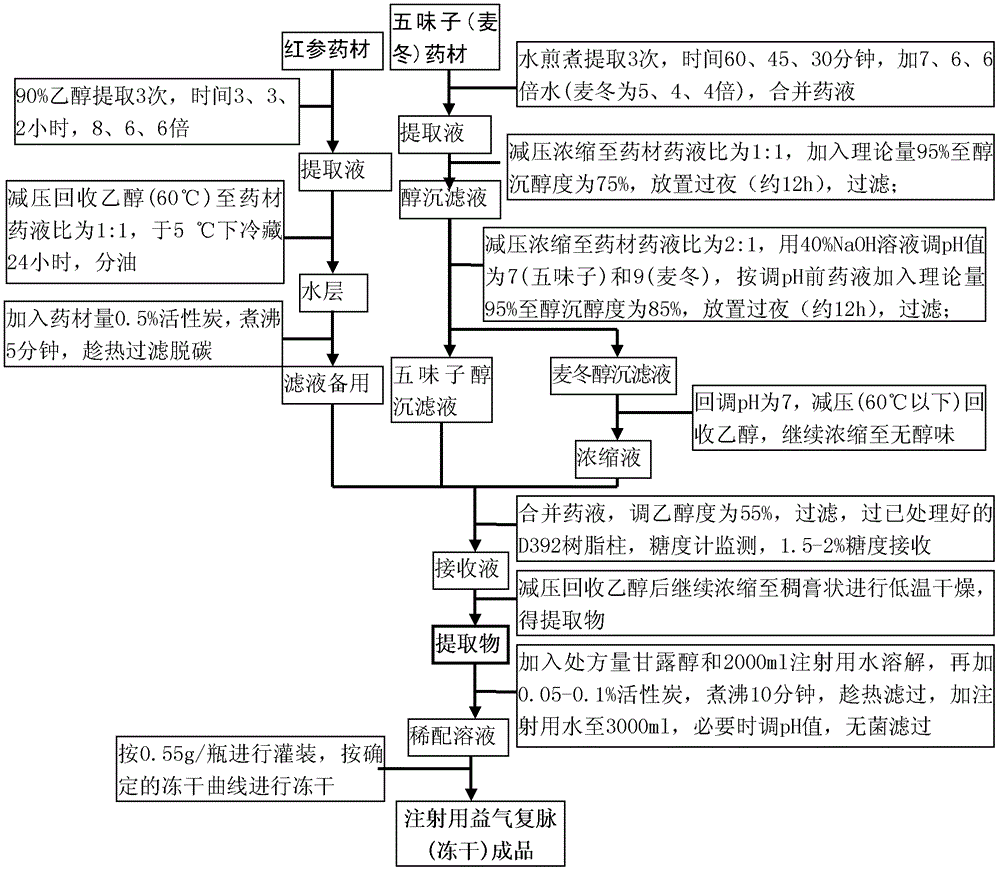
[0061] 步骤1,红参用90%乙醇回流提取三次,加90%乙醇分别为8、6、6倍;第一、二次分别为3小时,第三次2小时,合并提取液,减压回收乙醇至无醇味,加水至约500ml,冷置,分离除去上层油,水层煮至近沸,加入红参药材量的0.5%的活性炭,煮沸5分钟,趁热过滤除炭,滤液备用;
[0062] 步骤2,麦冬、五味子分别加水煎煮三次,麦冬、五味子加水量分别为5、4、4倍和7、6、6倍;第一次1小时,第二次45分钟,第三次30分钟,合并各自提取液,分别减压浓缩至药材药液比为1∶1,各加理论量乙醇至含醇量为75%,放置,滤过,滤液分别减压浓缩至药材药液比2∶1(必要时加纯化水调节),再分别加40%氢氧化钠溶液调节pH值分别为9(麦冬)、7(五味子),按调碱前药液量加入理论量乙醇至85%,放置,滤过;五味子滤液备用,
[0063] 步骤3,麦冬滤液加稀盐酸回调pH值为7,减压回收乙醇后至无醇味,备用;
[0064] 步骤4,将备用的麦冬浓缩液、红参滤液及五味子滤液合并(调醇浓度为55%),混匀,过滤,滤液以每小时约1.5~2倍树脂床体积的速度过D392树脂柱(药材树脂量比为1∶1),用糖度计监测流出液至有糖流出(约1~2%)时,接收流出液,并继续用55%乙醇冲洗,至接收的流出液与过柱的混合药液相当时,停止接收。接收的药液调pH值为6.5~7.0,于55~65℃减压回收乙醇后继续浓缩至稠膏状进行低温干燥,得提取物。
[0065] 步骤5,取处方量的提取物,与甘露醇混合(每瓶加入0.27~0.33g),加入加注射用水2000ml溶解,煮至近沸,加约0.05~0.1%的活性炭,加热煮沸10分钟,滤过,滤液用氢氧化钠溶液调pH值为6.0~7.0,加注射用水至总体积约3升,无菌滤过(0.22μm),灌装于西林瓶中,冷冻干燥,压塞、加盖,制成1000瓶,即得。
Embodiment 2
[0067] 注射用益气复脉冷冻干燥制剂,
[0068] 处方如下:
[0069]
[0070] 步骤1,红参用86%乙醇回流提取2次,加86%乙醇分别为8、6、倍;第一、二次分别为2小时,合并提取液,减压回收乙醇至无醇味,加水至约500ml,冷置,分离除去上层油,水层煮至近沸,加入红参药材量的0.5%的活性炭,煮沸5分钟,趁热过滤除炭,滤液备用;
[0071] 步骤2,麦冬、五味子分别加水煎煮2次,麦冬、五味子加水量分别为5、4、倍和7、6、倍;第一次1小时,第二次45分钟,合并各自提取液,分别减压浓缩至药材药液比为1∶1,各加理论量乙醇至含醇量为70%,放置,滤过,滤液分别减压浓缩至药材药液比2∶1(必要时加纯化水调节),再分别加40%氢氧化钠溶液调节pH值分别为9(麦冬)、7(五味子),按调碱前药液量加入理论量乙醇至80%,放置,滤过;五味子滤液备用,
[0072] 步骤3,麦冬滤液加稀盐酸回调pH值为7,减压回收乙醇后至无醇味,备用;
[0073] 步骤4,将备用的麦冬浓缩液、红参滤液及五味子滤液合并(调醇浓度约为50%),混匀,过滤,滤液以每小时约1.5~2倍树脂床体积的速度过D392树脂柱(药材树脂量比约为1∶1),用糖度计监测流出液至有糖流出(约1~2%)时,接收流出液,并继续用50%乙醇冲洗,至接收的流出液与过柱的混合药液相当时,停止接收。接收的药液调pH值为6.5~7.0,于55~65℃减压回收乙醇后继续浓缩至稠膏状进行低温干燥,得提取物。
[0074] 步骤5,取处方量的提取物,与甘露醇混合(每瓶加入0.27~0.33g),加入加注射用水2000ml溶解,煮至近沸,加约0.05~0.1%的活性炭,加热煮沸10分钟,滤过,滤液用氢氧化钠溶液调pH值为6.0~7.0,加注射用水至总体积约3升,无菌滤过(0.22μm),灌装于西林瓶中,冷冻干燥,压塞、加盖,制成1000瓶,即得。
Embodiment 3
[0076] 注射用益气复脉冷冻干燥制剂,
[0077] 处方如下:
[0078]
[0079] 步骤1,红参用94%回流提取4次,加94%乙醇分别为8、6、6、6倍;第一、二次分别为3小时,第三、四次2小时,合并提取液, 减压回收乙醇至无醇味,加水至约500ml,冷置,分离除去上层油,水层煮至近沸,加入红参药材量的0.5%的活性炭,煮沸5分钟,趁热过滤除炭,滤液 spare;
[0080]Step 2, decoct Ophiopogon japonicus and Schisandra chinensis with water for 4 times respectively, the amount of water added to Ophiopogon japonicus and Schisandra chinensis is 5, 4, 4, 4 times and 7, 6, 6, 6 times respectively; the first time is 1 hour, the second time is 45 Minutes, 30 minutes for the third and fourth times, combine the respective extracts, concentrate under reduced pressure until the ratio of medicinal materials to liquid is 1:1, add a theoretical amount of ethanol to the alcohol content of 80%, place, filter, and reduce the filtrates respectively Concentrate under pressure until the ratio of medicinal materials to liquid is 2:1 (add purified water to adjust if necessary), then add 40% sodium hydroxide solution to adjust the pH value to 9 (Ophiopogon ja...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com