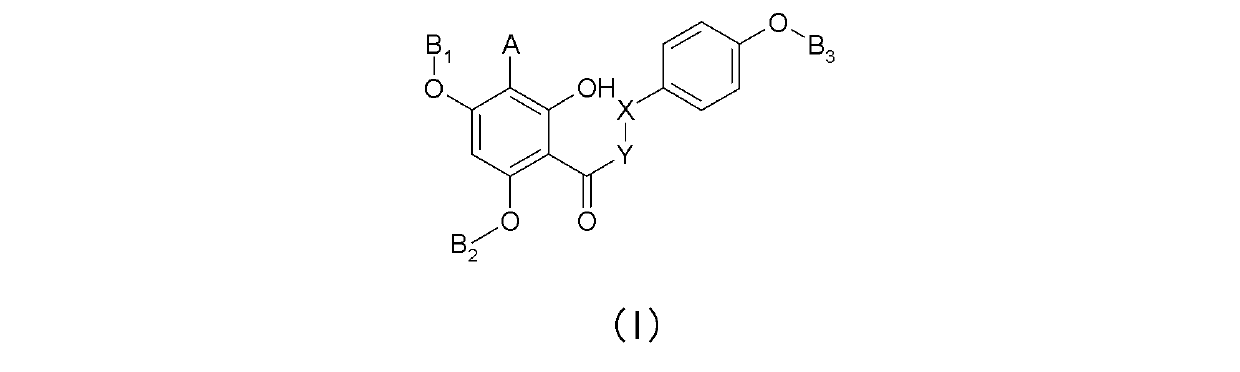
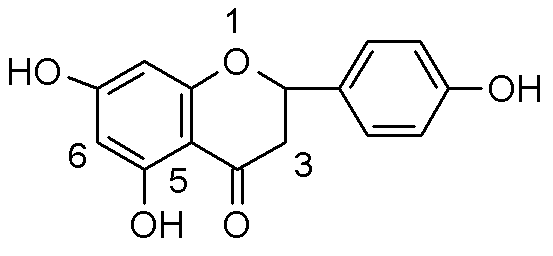
Substituted phloroglucinol derivatives and application thereof
A technology of phloroglucinol and derivatives, which is applied in the field of series substituted phloroglucinol derivatives, and achieves the effects of stable physical and chemical properties, simple synthesis process, and improvement of respiratory system function.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (E)-1-(2,4-Diallyloxy)-6-hydroxyphenyl)-3-(4-(allyl)phenyl)propene-1-one. (E)-1-(2,4-bis(allyloxy)-6-hydroxyphenyl)-3-(4-(allyloxy)phenyl) prop-2-en-1-one.
[0029]
[0030] Dissolve 5g of naringenin in 50ML of N,N-dimethylformamide (DMF), and then add 3eq of potassium carbonate K 2 CO 3 , the system was cooled to 0 degrees, and two equivalents of 2eq allyl bromide were added dropwise. After gradually rising to room temperature, react for 24 hours, add dropwise 600ML aqueous sodium chloride solution, and extract with ethyl acetate. The organic phase was washed three times with water, concentrated, and separated by silica gel column chromatography (petroleum ether: ethyl acetate = 20:1) to obtain 1.3 g of a light yellow solid. Melting point MP=55-58 degrees, thin layer chromatography: petroleum ether: ethyl acetate = 5:1, Rf=0.7, mass spectrum M+H= 393.3, yield = 18%.
[0031] NMR: 1 H-NMR(400Mhz,DMSO-d6): δ4.634(6H, dd, J1=17.6Hz; J2=4.8Hz, allyloxy-OCH2-),δ5.28...
Embodiment 2
[0035] 1-(2,4,6-Trihydroxy-3-propylphenyl)-3-4-hydroxyphenyl)propane 1-one.
[0036] 1-(2,4,6-trihydroxy-3-propylphenyl)-3-(4-hydroxyphenyl)propan-1-one.
[0037]
[0038] Dissolve 1 g of 6-allyl naringenin in 100 ml of ethanol. Add catalyst 10% Pd / C, 0.5g. Hydrogenation at normal pressure for 20 hours. The solid was removed by filtration, the filtrate was concentrated, and separated and purified by silica gel column chromatography (petroleum ether: ethyl acetate = 2:1) to obtain 0.11 g of a light red solid. Melting point MP=183-185 degrees. Thin layer chromatography: petroleum ether: ethyl acetate = 1:1, Rf = 0.3, mass spectrum M-H = 315.3, HPLC purity = 99%.
[0039] NMR: 1 H-NMR(400Mhz, DMSO-d6): δ0.854 (3H,t,J=7.2Hz,H-3''), δ1.405(2H,q,J1=7.2Hz, H-2'') , δ2.386(2H,t, J=7.6Hz, H-1''), δ2.761(2H, t, J=8Hz,H-2), δ3.224(2H,t,J=7.6Hz , H-3), δ5.999(1H, s, H-6), δ6.663(2H, d, J=8.4Hz, H-3',H-5'), δ7.015(1H, d , J=8.4Hz, H-2',H-6'), δ9.110(-9OH), δ10.201(-4'OH), δ10.516...
Embodiment 3
[0043] (E)-1-(4-propenyloxy)-2,6-dihydroxyphenyl)-3-(4-(propenyloxy)phenyl)propen-1-one.
[0044] (E)-1-(4-(allyloxy)-2,6-dihydroxyphenyl)-3-(4-(allyloxy)phenyl)prop-2-en-1-one.
[0045]
[0046] Dissolve 5g of naringenin in 50ML of N,N-dimethylformamide (DMF), then add 3eq equivalent of potassium carbonate K2CO3, cool the system to 0 degrees, and dropwise add two equivalents of 2eq allyl bromide. After gradually rising to room temperature, react for 24 hours, add dropwise 600ML aqueous sodium chloride solution, and extract with ethyl acetate. The organic phase was washed three times with water, concentrated, and separated by silica gel column chromatography, petroleum ether: ethyl acetate = 10:1 to obtain 2 g of a light yellow mixture. Recrystallization from dichloromethane gave 0.6 g of a solid. Melting point MP=121-125 degrees, thin layer chromatography: Rf=0.7, petroleum ether: ethyl acetate = 2:1, Rf=0.7, mass spectrum M+H= 393.3.
[0047] NMR: 1 H-NMR(400Mhz, DMSO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com