Preparation method of sulfate surfactant containing fluoride anion and use thereof
A surfactant and sulfate-based technology, applied in the preparation of sulfonamides, chemical instruments and methods, organic chemistry, etc., can solve problems such as no literature reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] Example 1: N-methyl-N-hydroxyethyl-4-perfluoro-(1,3-dimethyl-2-isopropyl)-1-butenyloxybenzenesulfonamide
[0022] In a 100 mL four-necked flask with a reflux condenser, 4-perfluoro-(1,3-dimethyl-2-isopropyl)-1-butenyloxybenzenesulfonyl chloride (3.11 g, 0.005 mol) was dissolved in 20 mL of acetonitrile and stirred to dissolve. 2-Methylaminoethanol (0.75 g, 0.01 mol) was added dropwise at room temperature for about 0.5 h. After the dropwise addition was completed, the reaction was stirred at room temperature for 3.0 h. After the reaction was completed, the solvent was removed under reduced pressure, washed with 3×10 mL of water, and the resulting white turbid viscous liquid was dried and recrystallized to obtain a white solid powder, about 3.01 g, with a yield of 92%. IR (cm ?1 ): υ(O-H) 3497.1,υ(C-H)2963.2,υ(C=C) 1592.0,1491.4,υ(S=O) 1012.1,υ(C-F)1287.2,1185.0,1127.1,980.1; 1 H-NMR ((CD 3 ) 2 SO)δ: 7.92(d,2H,ArH),7.37(d,2H,ArH),3.51(t,2H,CH 2 ),3.03(t,2H,CH 2 ),...
example 2
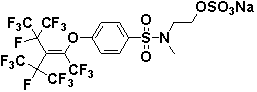
[0023] Example 2: Preparation of [N-methyl-(4-perfluoro-(1,3-dimethyl-2-isopropyl)-1-butenyloxy)benzenesulfonamido]ethyl sulfate
[0024] In a 100 mL four-neck flask, add N-methyl-N-hydroxyethyl-4-perfluoro-(1,3-dimethyl-2-isopropyl)-1-butenyloxybenzenesulfonate Amide (3.30 g, 0.005mol) was dissolved in 40 mL of carbon tetrachloride, stirred and heated to reflux, and chlorosulfonic acid (1.16 g, 0.01mol) was slowly added dropwise for about 30 min, and the reaction was continued for 6 h. After the reaction, let stand to cool to room temperature, remove the solvent under reduced pressure after decolorization, add to 10% NaOH solution, stir, and neutralize to obtain a white viscous liquid, add 50mL saturated saline, precipitate a white solid, filter, and dry Obtain [N-methyl-(4-perfluoro-(1,3-dimethyl-2-isopropyl)-1-butenyloxy) benzenesulfonamido] sodium ethyl sulfate, yield 80% . IR (cm -1 ): υ(N-H)3446.3,υ(C=C)1626.6,1590.9,1491.5,υ(C-O)1241.8;υ(S=O)1014.2,υ(C-F)1240.8,979.5...
example 3
[0025] Example 3 Surface tension test (Wilhelmy method)
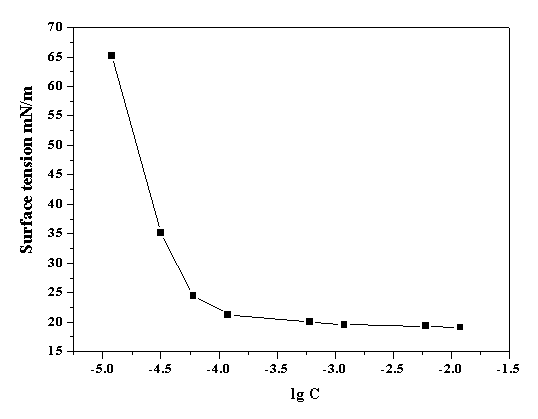
[0026] The hanging piece method, also known as the Wilhelmy method, uses cover glass, mica piece, filter paper, platinum foil, and a vertical plate is inserted into the test liquid, so that the bottom edge is in contact with the liquid surface, and the surface tension required when the hanging piece is separated from the liquid is measured. The maximum pulling force F. This method is intuitive and reliable, does not require a correction factor, which is different from other detachment methods, and can also measure liquid-liquid interfacial tension. by attaching figure 1 , it can be concluded that its critical micelle concentration is 4.8×10 -4 mol / L, the surface tension of its aqueous solution (γ CMC ) is 19.5mN / m (the surface tension of pure water is 71.0 mN / m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com