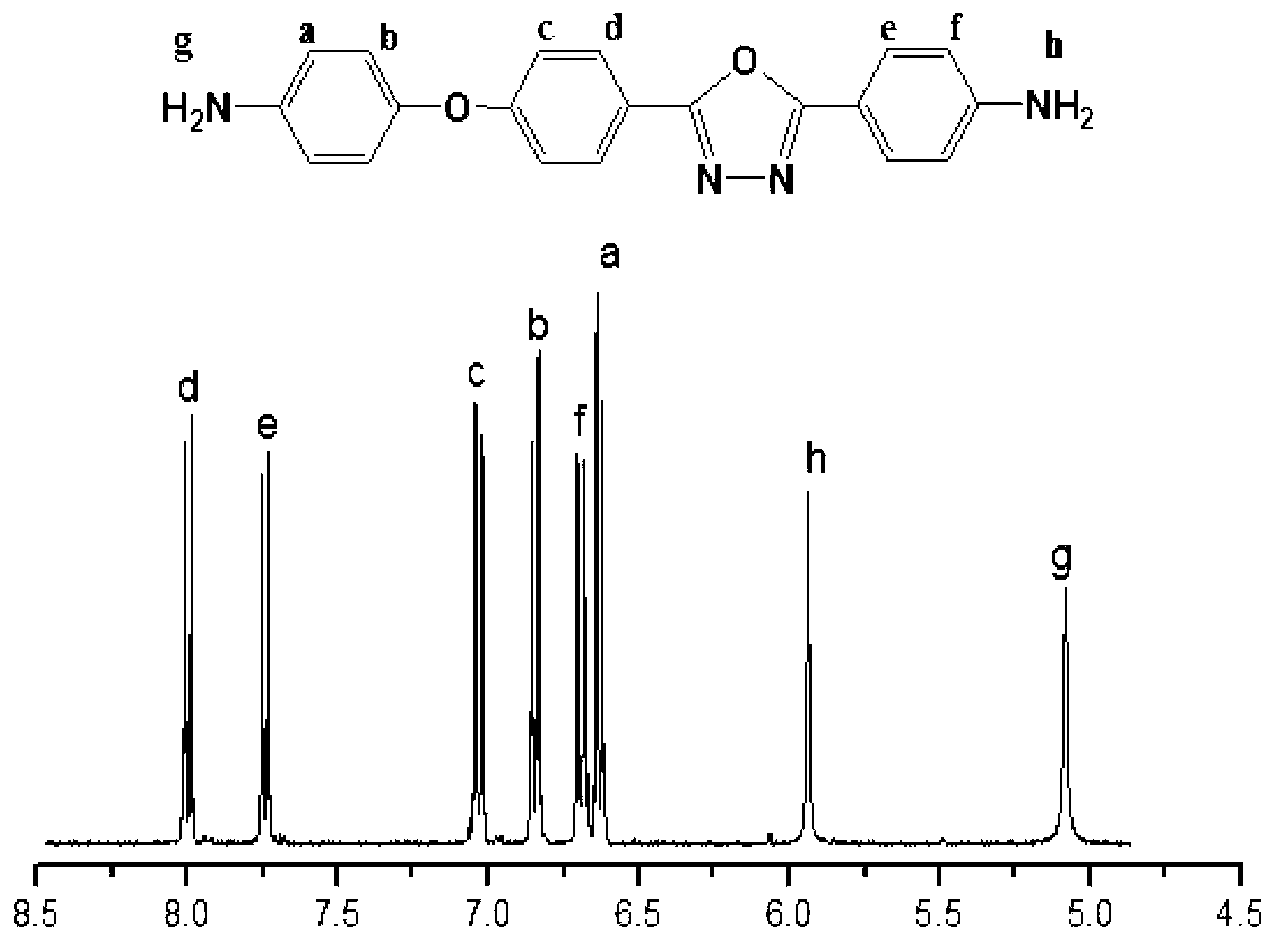
Asymmetric aromatic diamine containing 1,3,4-oxadiazole structure and preparation method thereof
An aromatic diamine, asymmetric technology, applied in the field of chemical intermediate synthesis, can solve the problems of high rigidity, poor polymer toughness and solubility, and achieve the effect of good heat resistance and excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
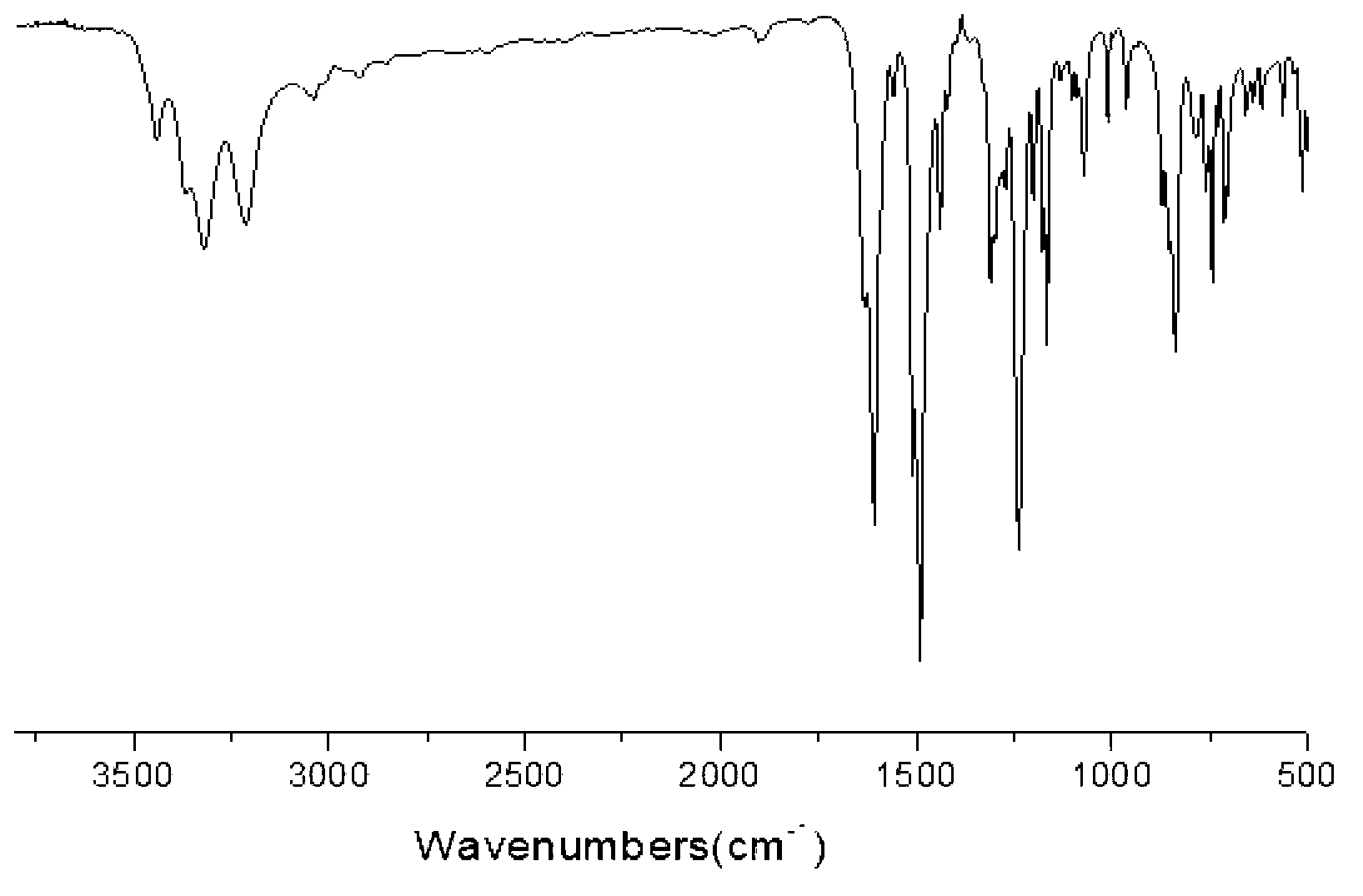
[0033] (A) Add 0.1mol of p-chloronitrobenzene, 0.12mol of anhydrous potassium carbonate, 0.11mol of p-cresol, and 60ml of N,N-dimethylformamide into the reaction kettle, and react 12 at 120°C hour; filtered while hot, cooled the filtrate, added cold water, precipitated, filtered, washed the filter cake three times with distilled water, filtered, and dried in vacuum to obtain light yellow 4-methyl-4'-nitrodiphenyl ether, the yield was 96.3 %, the purity determined by HPLC is 99.6%, and the melting point is 67°C. FT-IR (KBr, cm -1 ): 2946, 2920 (-CH 3 ), 1509, 1343 (-NO 2 ), 1248, 1111 (Ar-O).
[0034] (B) Add 0.1mol of 4-methyl-4'-nitrodiphenyl ether, 0.5mol of sodium hydroxide, 50ml of water, and 100ml of pyridine into the reaction kettle, stir to dissolve, and then add 0.5mol of potassium permanganate Add to the reaction kettle in batches, react at 90°C for 10 hours, cool, filter, acidify the filtrate with acid, a white solid precipitates, filter and dry to obtain 4-p-nit...
Embodiment 2
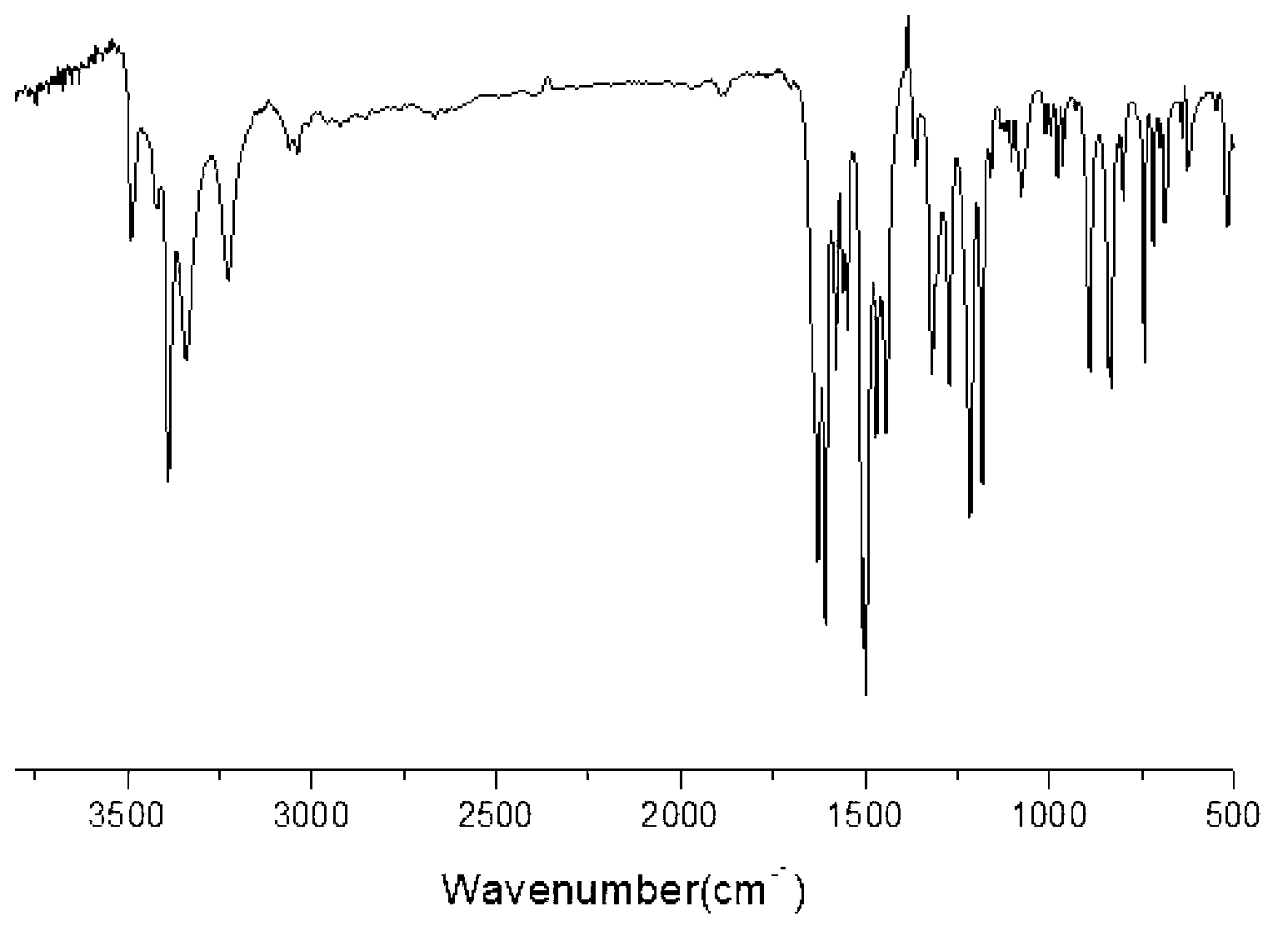
[0041] (A) Add 0.1mol of p-chloronitrobenzene, 0.12mol of anhydrous potassium carbonate, 0.11mol of m-cresol, and 50ml of N,N-dimethylformamide into the reaction kettle, and react 10 at 125°C hour; filtered while hot, cooled the filtrate, added cold water, precipitated, filtered, washed the filter cake three times with distilled water, filtered, and vacuum-dried to obtain light yellow 3-methyl-4'-nitrodiphenyl ether, yield 97.1 %, the purity determined by HPLC is 99.3%, and the melting point is 62°C. FT-IR (KBr, cm -1 ): 2923, 2951 (-CH 3 ), 1501, 1345 (-NO 2 ), 1250, 1108 (Ar-O).
[0042] (B) Add 0.05mol of 4-methyl-3'-nitrodiphenyl ether, 0.3mol of sodium hydroxide, 30ml of water, and 70ml of pyridine into the reaction kettle, stir to dissolve, and then add 0.3mol of potassium permanganate Add to the reactor in batches, react at 95°C for 9 hours, cool, filter, acidify the filtrate with acid, a white solid precipitates, filter and dry to obtain 3-p-nitrophenoxybenzoic aci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com