Cephalosporin ester drug particle in non-gel state after meeting water, and preparation method and application of drug particle
A cephalosporin, non-gel technology, applied in the field of pharmaceutical preparations, can solve problems such as slow dissolution and gelation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0026] Prepare the drug granules shown in Examples 1-3 with the following method and the formula shown in Table 1: sieve the cefuroxime axetil raw material, micropowder silica gel, anti-gelling auxiliary agent, and disintegrant respectively, and then sieve the above-mentioned The final raw and auxiliary materials are mixed evenly, pressed into large pieces, and then sieved and granulated. The details are shown in Table 1:
[0027] Table 1 Statistical Table of Formulation of Embodiment 1-3 (parts by weight)
[0028] .
Embodiment 4-6
[0030] Examples 4-6 adopt the following formula: in parts by weight, 100 parts of cefuroxime axetil, 3.2 parts of micropowdered silica gel, 3.2 parts of stearic acid as an anti-gelling agent, and 14 parts of croscarmellose sodium as a disintegrating agent.
[0031] The preparation method is as follows: mix part of cefuroxime axetil raw material (premixed cefuroxime axetil raw material) with part of micropowder silica gel (premixed micropowder silica gel), sieve, and then mix with other cefuroxime axetil raw materials that have passed through a 40-mesh sieve respectively , micropowder silica gel, anti-gelling agent stearic acid, and disintegrating agent are mixed, pressed into large tablets, and then granulated through a 24-mesh sieve. The details are shown in Table 2:
[0032] Table 2 Statistical table of premixed cefuroxime axetil raw materials and premixed micropowder silica gel in Examples 4-6 (parts by weight)
[0033] .
Embodiment 7-12
[0049] With reference to the preparation method of Example 2, the drug granules of Examples 7-12 were prepared, and the bulk density and dissolution rate of the granules were measured according to the methods shown in Table 5. The details are shown in Table 5:
[0050] Table 5 The formula of embodiment 7-12 and the statistical table of dissolution test situation (parts by weight)
[0051] .
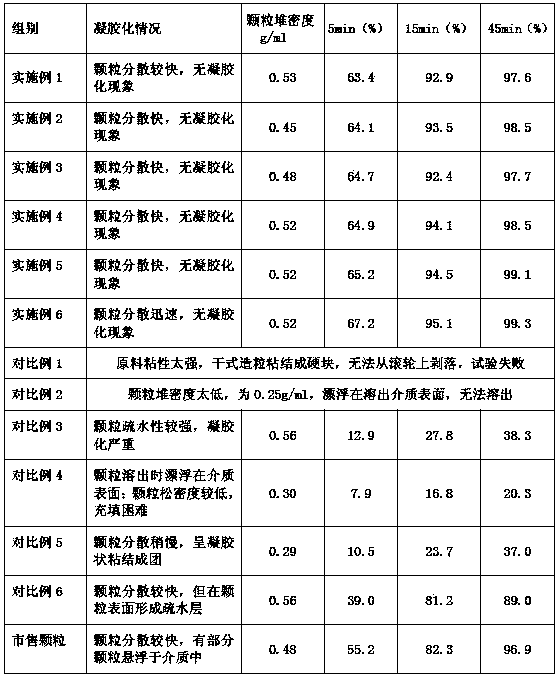
[0052] Analysis of the above measurement data shows that the formula and preparation method of the present invention can be applied to other cephalosporin ester drugs such as cefpodoxime axetil, cefditoren axetil and cefditoren neopentyl pivoxil, and have a wide range of uses. At the same time, the bulk density of the obtained granules is also in the range of 0.4-0.6g / ml, and they disperse quickly in the dissolution medium without gelation. The average dissolution rate in 15 minutes is over 93%, and the average dissolution rate in 45 minutes is over 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com