Novel synthesis method of polyfluorene derivative and application in immunosensor for detecting tumor marker
A derivative and fluorescence sensor technology, applied in the field of immune marker synthesis and immune detection, can solve the problems of unfavorable popularization, long time consumption, complicated operation, etc., and achieve the effect of reducing impact, improving biocompatibility, and high fluorescence efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] A method for preparing a flow injection fluorescent sensor for detecting immune markers, comprising the following steps:
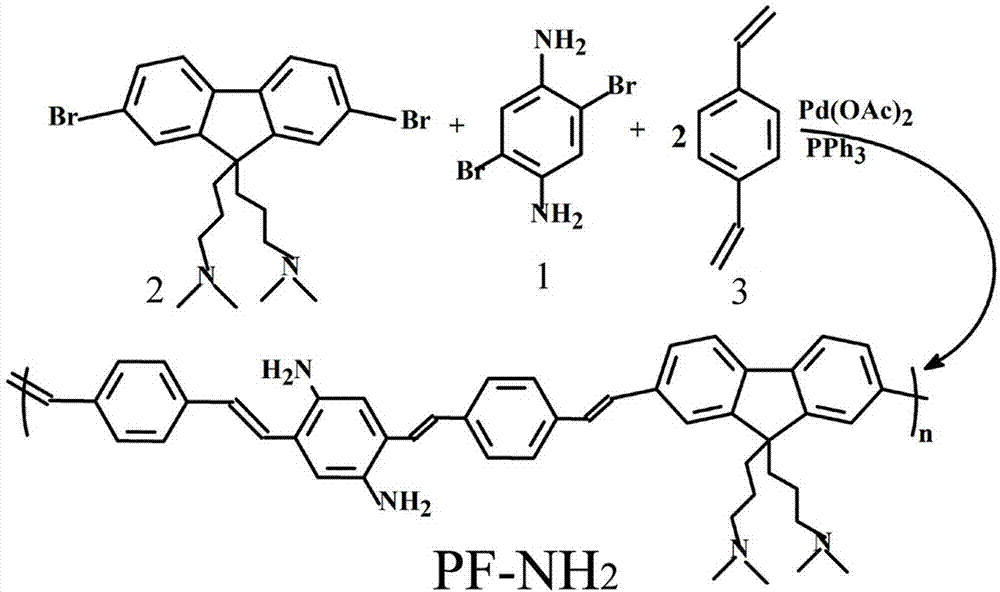
[0044] (1) Synthesis of polyfluorene derivatives (PF-NH 2 );
[0045] (2) Synthesis of silica-coated polyfluorene derivatives (PF-NH 2 SiO 2 );
[0046] (3) Modify the secondary antibody in PF-NH 2 SiO 2 ;
[0047] (4) Prepare gold nanoparticles (AuNPs), ferroferromagnetic particles (Fe 3 o 4 ) Gold nanoparticles coated iron oxide (Fe 3 o 4 Au);
[0048] (5) Modify the primary antibody on Fc 3 o 4 Au surface;
[0049] (6) Fabricate flow injection fluorescent flow cells using polymethyl methacrylate (PMMA);
[0050] (7) Combine the prepared chemiluminescence sensor with a flow injection chemiluminescence instrument to detect immune markers.
[0051] The preparation of the silicon dioxide-coated polyfluorene derivative and the silicon dioxide-coated ferric oxide of the present invention comprises the following steps:
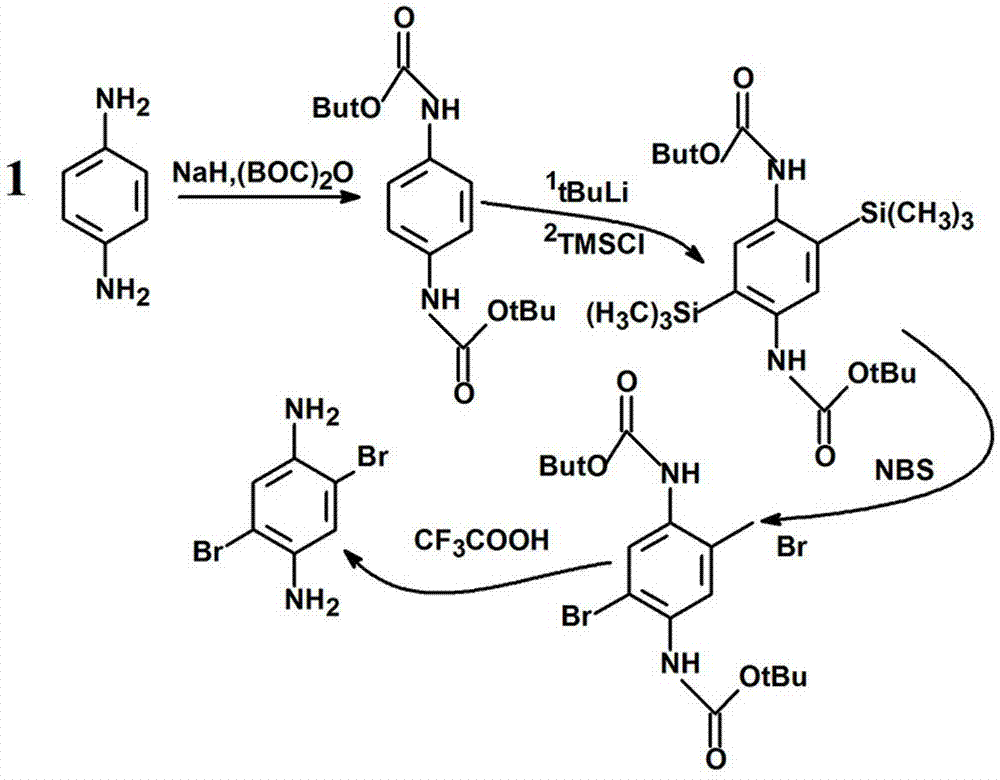
[0052] (1) 1,4-...
Embodiment 1
[0071] Example 1 (Carbohydrate markers, such as CA19-9)
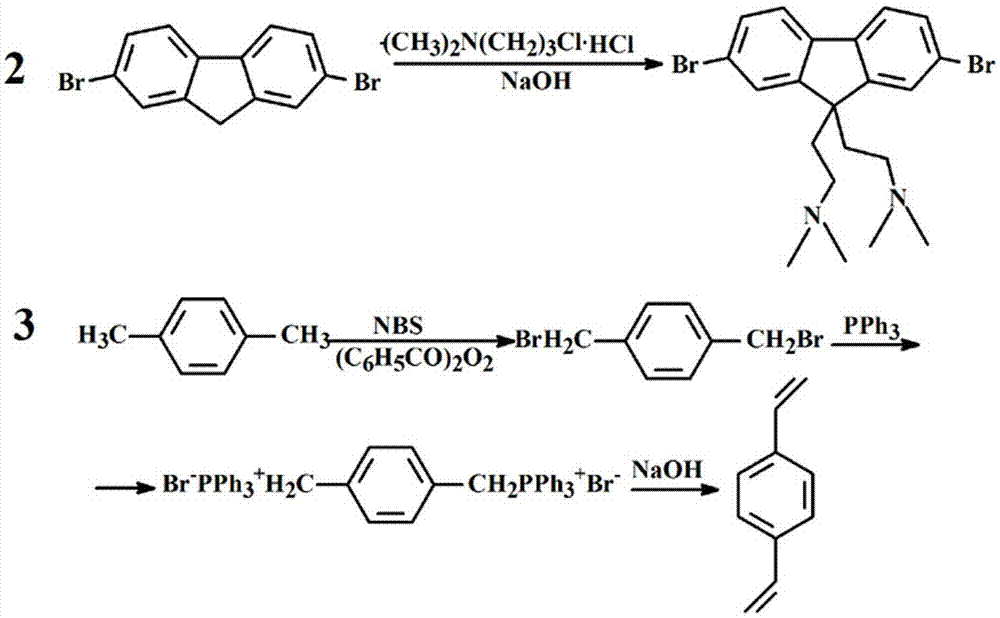
[0072] (1) First synthesize three monomers: 1,4-dibromo-p-phenylenediamine (compound 1), 2,7-dibromo-9,9-bis(3′-(N, N′-dimethylamino )Propyl)-fluorene (compound 2) and p-divinylbenzene (compound 3). Polymerization into PF-NH by Heck 2 Synthesis.
[0073] (2) The PF-NH prepared in (1) 2 Prepare solution (1 nM in DMF). Add 1 mL PF-NH to a 100 mL one-necked bottle 2 solution, 8 mL of ethanol, stirred to disperse evenly. Then 0.1 mL of ammonia solution (25%) was added, and 10 μL of TEOS solution was stirred for 30 min. Continue to add 10 μL of TEOS solution and stir for 30 min until the total amount of TEOS solution added is 50 μL. After the reaction, at 10000 r min -1 Centrifuge at a rotating speed, wash the precipitate with ethanol, and repeat three times. Dry in a vacuum oven at 50 °C for 5 h to obtain PF-NH 2 SiO 2 .
[0074] (3) Add the PF-NH prepared in (2) into a small 10 mL beaker 2 SiO 2 0.02 g, 1 ...
Embodiment 2
[0083] Example 2 (Hormones, such as HCG)
[0084] (1) First synthesize three monomers: 1,4-dibromo-p-phenylenediamine (compound 1), 2,7-dibromo-9,9-bis(3′-(N, N′-dimethylamino )Propyl)-fluorene (compound 2) and p-divinylbenzene (compound 3). Polymerization into PF-NH by Heck 2 Synthesis.
[0085] (2) The PF-NH prepared in (1) 2 Prepare solution (1 nM in DMF). Add 1 mL PF-NH to a 100 mL one-necked bottle 2 solution, 8 mL of ethanol, stirred to disperse evenly. Then 0.1 mL of ammonia solution (25%) was added, and 10 μL of TEOS solution was stirred for 30 min. Continue to add 10 μL of TEOS solution and stir for 30 min until the total amount of TEOS solution added is 50 μL. After the reaction, at 10000 r min -1 Centrifuge at a rotating speed, wash the precipitate with ethanol, and repeat three times. Dry in a vacuum oven at 50 °C for 5 h to obtain PF-NH 2 SiO 2 .
[0086] (3) Add the PF-NH prepared in (2) into a small 10 mL beaker 2 SiO 2 0.02 g, 1 mL of CDI solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com