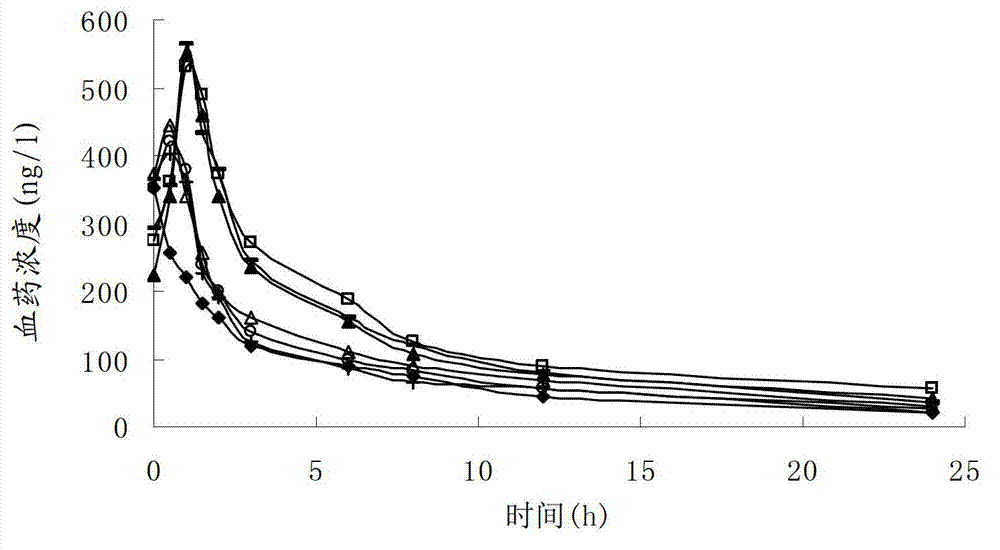
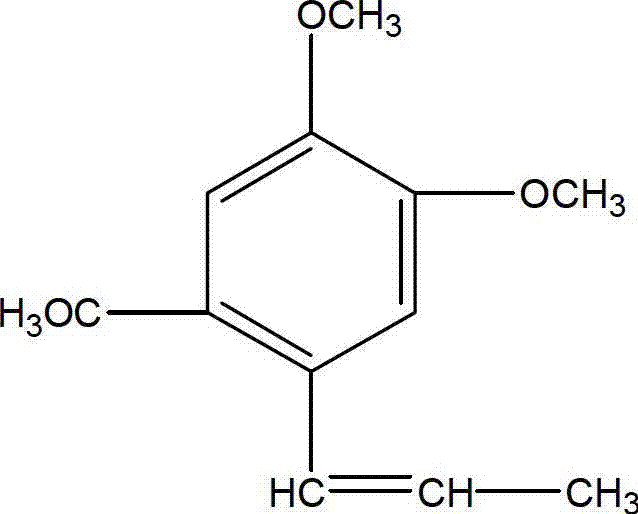
Asarone lipidosome injection
A technology of caprylyl and injection, which is applied in the directions of liposome delivery, medical preparations of inactive ingredients, freeze-drying delivery, etc., can solve the problems of poor stability, low bioavailability, and low encapsulation rate of main drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1 asarone liposome freeze-dried powder injection
[0054] prescription:
[0055]
[0056] Preparation Process:
[0057] (1) Dissolve 8g of asarone, 50g of soybean phosphatidylinositol, 30g of cholesterol and 40g of Span-60 in 1000ml of chloroform, and then put it in an eggplant-shaped bottle and rotate it until a uniform film is formed;
[0058] (2) Add 1000ml of phosphate buffer solution with a pH of 6.8 and an appropriate amount of glass beads to the eggplant-shaped bottle and stir for 10 minutes, then stir in a water bath at 30°C for 2 hours to obtain asarone liposomes;
[0059] (3) Add 40g of trehalose to the asarone liposomes prepared above, use a probe to sonicate for 10 minutes, dilute to 2000ml with water for injection, filter through a 0.22um microporous membrane, sub-package, freeze-dry, roll Cap, made 1000 bottles of asarone liposome freeze-dried powder injection.
Embodiment 2
[0060] Embodiment 2 Asarone liposome injection
[0061] prescription:
[0062]
[0063] Preparation Process:
[0064] (1) Dissolve 16g of asarone, 80g of soybean phosphatidylinositol, 40g of cholesterol and 60g of Span-60 in 1500ml of chloroform, and then put it in an eggplant-shaped bottle and rotary evaporate until a uniform film is formed;
[0065] (2) Add 1500ml of phosphate buffer solution with a pH of 6.8 and an appropriate amount of glass beads to the eggplant-shaped bottle and stir for 10 minutes, then stir in a water bath at 30°C for 2 hours to obtain asarone liposomes;
[0066] (3) Add 45g of sodium chloride to the asarone liposomes prepared above, ultrasonicate for 10 minutes with a probe, make the volume to 5000ml with water for injection, filter through a 0.22um microporous membrane, freeze quickly, and then return to room temperature, potting, and sterilization to prepare 1000 Asarone liposome injections.
Embodiment 3
[0067] Embodiment 3 asarone liposome injection
[0068] prescription:
[0069]
[0070] Preparation Process:
[0071] (1) Dissolve 24g of asarone, 60g of soybean phosphatidylinositol, 150g of cholesterol and 100g of Span-60 in 4000ml of chloroform, then place it in an eggplant-shaped bottle and rotate it until a uniform film is formed;
[0072] (2) Add 4000ml of phosphate buffer solution with a pH of 6.8 and an appropriate amount of glass beads to the eggplant-shaped bottle and stir for 10 minutes, then stir in a water bath at 30°C for 2 hours to obtain asarone liposomes;
[0073] (3) Add 90g of sodium chloride to the asarone liposomes prepared above, ultrasonicate with a probe for 10 minutes, dilute to 10000ml with water for injection, filter through a 0.22um microporous membrane, freeze quickly, and then return to room temperature, potting, and sterilization to prepare 1000 Asarone liposome injections.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com