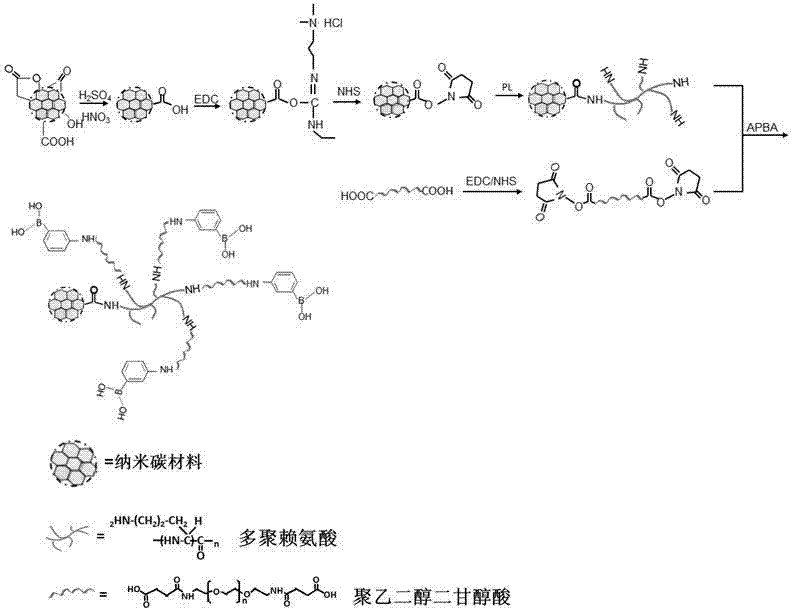
Aminophenylboronic acid surface-modified nano-carbon material, as well as preparation method and application thereof
A nanocarbon material and surface modification technology, applied in the preparation method of peptides, nanotechnology for materials and surface science, nanocarbon, etc., can solve the problems of reduced possibility, signal inhibition, difficult analysis, etc. The effect of dispersibility and stability, strong specificity, good practical value and application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1. Modification of aminophenylboronic acid on the surface of nanodiamond:
[0029] (1) Pretreatment of nano-diamonds: 1-5g nano-diamonds are ultrasonically dispersed in 10-50mL H 2 SO 4 / HNO 3 (9:1-8:2 (v / v)) solution, stir at room temperature for 12-24 hours, wash with water until neutral; as for 0.1-0.5M NaOH aqueous solution, stir at 90-95°C for 2-4 hours, wash with water Cleaning; Stir in 0.1-0.5M HCl solution at 90-95°C for 2-4 hours, wash with water, and dry to obtain acidified / activated nano-diamonds; the above treatment process makes the surface generate carboxyl functional groups.
[0030] (2) Disperse 0.5-1g acidified / activated nano-diamonds in 2-(N-morpholino)ethanesulfonic acid buffer (100-200mM, pH 6.1). Ultrasonic dispersion for 2-5 minutes, then quickly add 5-10mL 60-80mg / mL N-ethyldimethylaminopropylcarbodiimide hydrochloride, continue ultrasonic dispersion for 10-30min, after the reaction is over, collect nano-diamonds by centrifugation ,...
Embodiment 2
[0033] Example 2. Modification of aminophenylboronic acid on the surface of graphene:
[0034] (1) Pretreatment of graphene: 1-5g graphene is ultrasonically dispersed in 10-50mL H 2 SO 4 / HNO 3 (9:1-8:2 (v / v)) solution, stir at room temperature for 12-24 hours, wash with water until neutral; as for 0.1-0.5M NaOH aqueous solution, stir at 90-95°C for 2-4 hours, wash with water Cleaning; stir in 0.1-0.5M HCl solution at 90-95°C for 2-4 hours, wash with water, and dry to obtain graphene oxide; the above treatment process makes the surface produce carboxyl functional groups.
[0035] (2) Disperse 0.5-1g graphene oxide in 2-(N-morpholino)ethanesulfonic acid buffer (100-200mM, pH 6.1) dissolved in 5-10mL 60-80mg / mL N-hydroxysuccinimide ). Ultrasonic dispersion for 2-5 minutes, then quickly add 5-10mL 60-80mg / mL N-ethyldimethylaminopropylcarbodiimide hydrochloride, continue ultrasonic dispersion for 10-30min, after the reaction is completed, the activated graphene, discard the ...
Embodiment 3
[0038] Example 3. Modification of aminophenylboronic acid on the surface of carbon nanotubes:
[0039] (1) Pretreatment of carbon nanotubes: 1-5g carbon nanotubes are dispersed in 10-50mL H 2 SO 4 / HNO 3 (9:1-8:2 (v / v)) solution, stir at room temperature for 12-24 hours, wash with water until neutral; as for 0.1-0.5M NaOH aqueous solution, stir at 90-95°C for 2-4 hours, wash with water Cleaning; stir in 0.1-0.5M HCl solution at 90-95°C for 2-4 hours, wash with water, and dry to obtain oxidized / acidified carbon nanotubes; the above treatment process makes the surface produce carboxyl functional groups.
[0040] (2) Disperse 0.5-1g of oxidized / acidified carbon nanotubes in 2-(N-morpholino)ethanesulfonic acid buffer (100-200mM) dissolved in 5-10mL of 60-80mg / mL N-hydroxysuccinimide , pH 6.1). Ultrasonic dispersion for 2-5 minutes, then quickly add 5-10mL 60-80mg / mL N-ethyldimethylaminopropylcarbodiimide hydrochloride, continue ultrasonic dispersion for 10-30min, after the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com