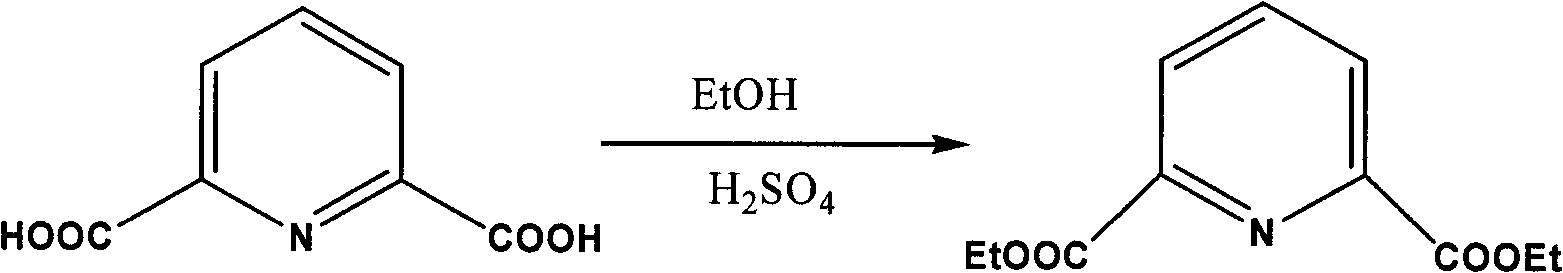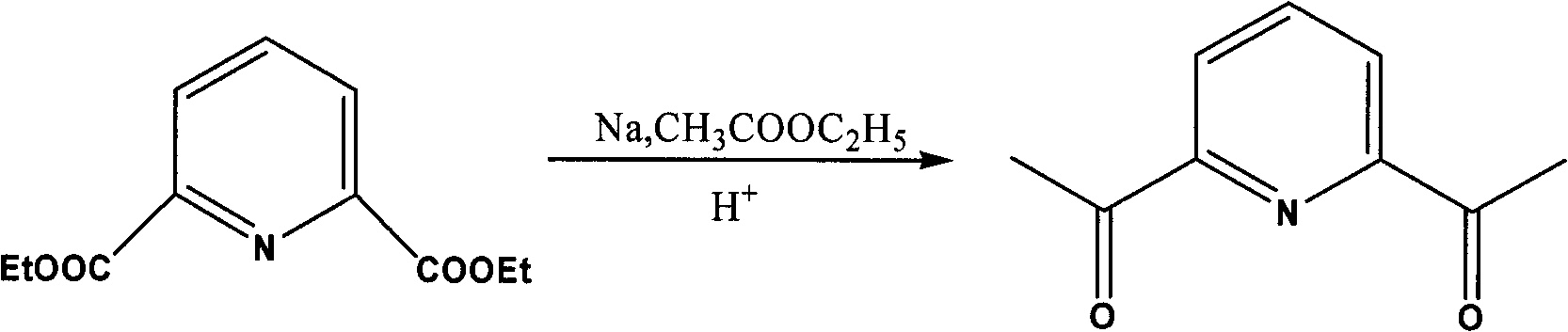Synthesis process of 2,6-diacetyl pyridine
A technique for the synthesis of diacetylpyridine, which is applied in 2 fields, can solve the problems of harsh process conditions, cumbersome operation, and long time consumption, and achieve the effects of easy post-processing, few synthesis steps, and short time consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] Processing step of the present invention is:
[0019] 1. Synthesis of ethyl 2,6-pyridinedicarboxylate
[0020] The reaction equation is:
[0021]
[0022] 2. Synthesis of 2,6-diacetylpyridine
[0023] The reaction equation is:
[0024]
[0025] In the second step reaction, the present invention selects metallic sodium as the base, and excess sodium can remove trace amounts of water and ethanol in the system, which greatly simplifies the operation steps and facilitates the reaction.
[0026] The present invention selects the addition amount of metal sodium as an influencing factor of the reaction yield, as shown in Table 1.
[0027] Table 1 Sodium and 2, the influence of the mass ratio of 6-pyridinedicarboxylate on the reaction yield
[0028] n:n
1∶1
2∶1
3∶1
4∶1
5∶1
6∶1
Yield / %
15.3
35.5
51.7
66.6
76.7
73.5
[0029] In the table, n:n is the ratio of the amount of so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com