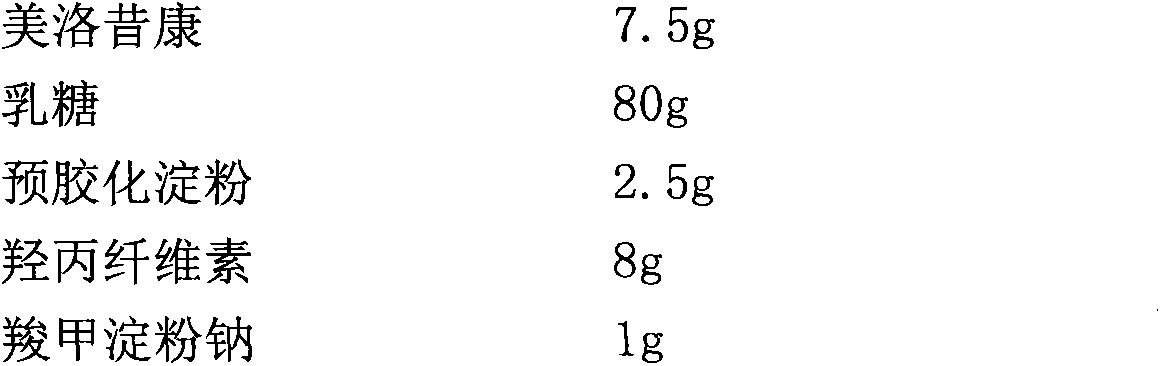Meloxicam pharmaceutical composition and preparation method thereof
A technology for meloxicam and composition, which is applied in the field of meloxicam pharmaceutical composition and its preparation, can solve the problems affecting the application of meloxicam, poor stability and the like, and achieves prescription and process optimization, resistance to Strong abrasiveness and beautiful appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
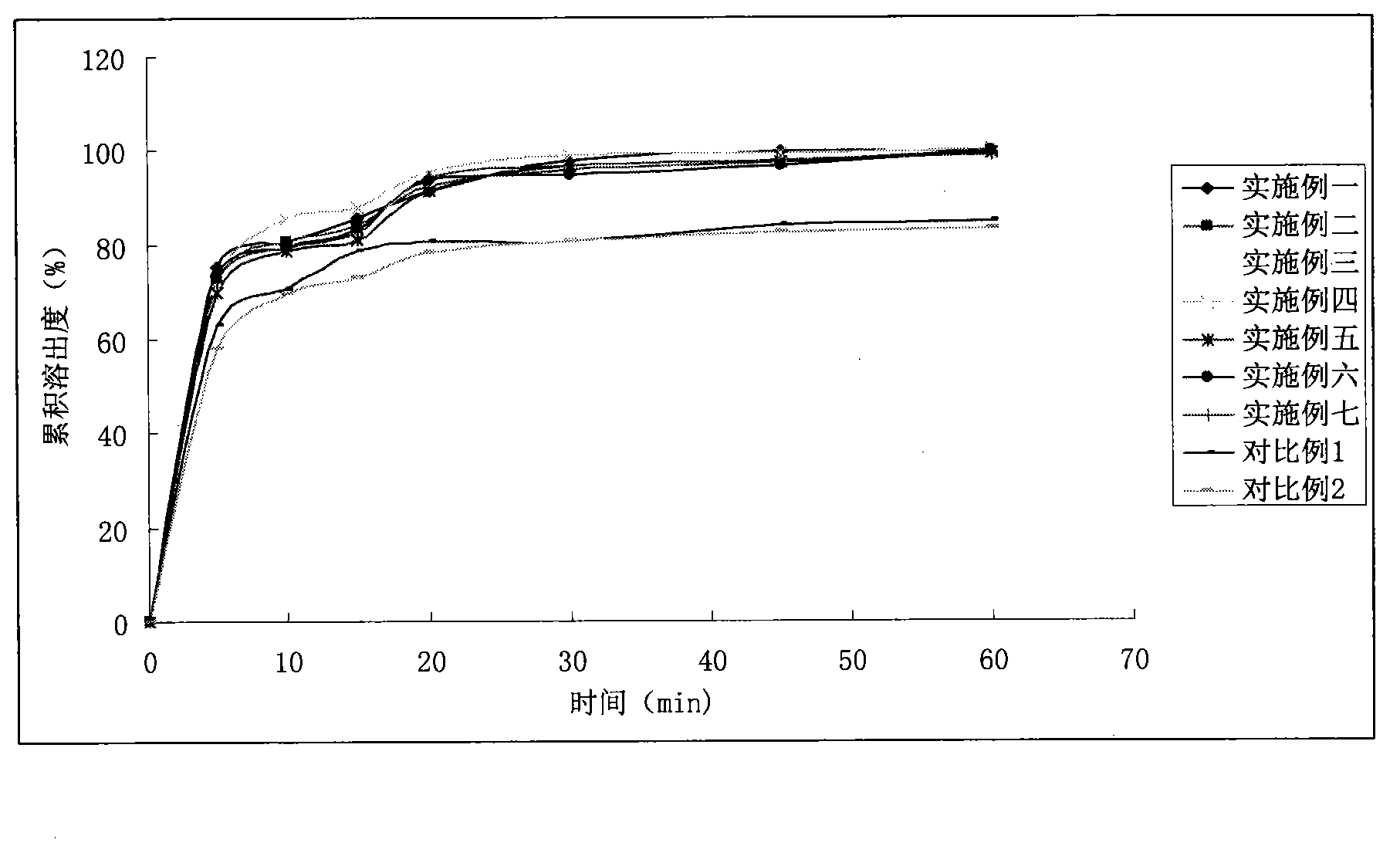
Image
Examples
Embodiment 1
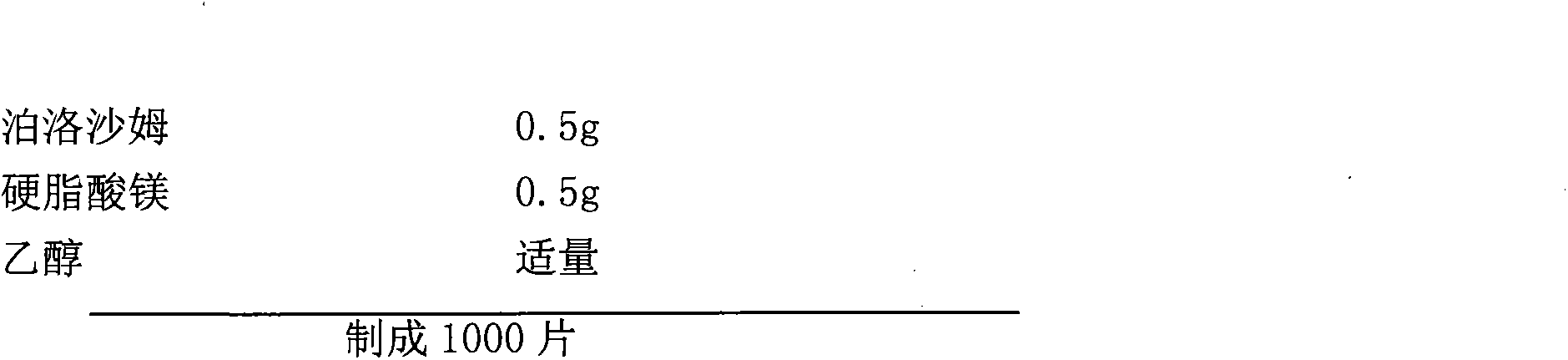
[0018] Raw material formula:
[0019]
[0020]
[0021] Preparation:
[0022] 1) Dissolve poloxamer in 5 times of ethanol to make a poloxamer solution; add pregelatinized starch to purified water to make a pregelatinized starch solution with a concentration of 8%; add the pregelatinized starch solution to Poloxamer solution, and add purified water to make a mixed solution A with a mass concentration of 10%.
[0023] 2) Pass the meloxicam through a 100-mesh sieve, pass the lactose through a 80-mesh sieve, mix the sieved meloxicam and lactose, add hydroxypropyl cellulose and mix to form a mixture B;
[0024] 3) Add the mixed solution A to the mixture B, stir evenly, and make particles C with a particle size of 24 mesh;
[0025] 4) Dry the granules C at 80°C for 15 minutes, add sodium starch glycolate and magnesium stearate after drying, mix them, and press at a pressure of 10KN for 1 hour to form tablets.
Embodiment 2
[0027] Raw material formula:
[0028]
[0029] Preparation:
[0030] 1) Dissolve poloxamer in 8 times of ethanol to make poloxamer solution; add pregelatinized starch to purified water to make pregelatinized starch solution with a concentration of 15%; add pregelatinized starch solution to Poloxamer solution, and add purified water to make a mixed solution A with a mass concentration of 12%.
[0031] 2) Pass the meloxicam through a 100-mesh sieve, pass the lactose through a 80-mesh sieve, mix the sieved meloxicam and lactose, add hydroxypropyl cellulose and mix to form a mixture B;
[0032] 3) Add the mixed solution A to the mixture B, stir evenly, and make particles C with a particle size of 24 mesh;
[0033] 4) Dry the granule C at 80°C for 15 minutes, add sodium starch glycolate and magnesium stearate after drying, and press it under a pressure of 10KN for 1 hour to form a tablet.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com