Medicine composition containing fructose sodium diphosphate compound
A technology of sodium fructose diphosphate and its composition, which is applied in the field of medicine and can solve problems such as pain discomfort, pain, and vascular smooth muscle spasm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1 sodium fructose diphosphate pharmaceutical composition (injection)
[0018] Formula 1: 30g of sodium fructose diphosphate, 0.5g of sodium bisulfite, 30g of mannitol, and 500ml of water for injection.
[0019] Formula 2: 10 g of sodium fructose diphosphate, 0.1 g of sodium bisulfite, 10 g of mannitol, and 100 ml of water for injection.
[0020] Formula 3: 50 g of sodium fructose diphosphate, 1 g of sodium bisulfite, 50 g of mannitol, and 1000 ml of water for injection.
[0021] Formula 4: sodium fructose diphosphate 20g, sodium bisulfite 0.8g, mannitol 40g, water for injection 200ml.
[0022] Formula 5: 35g of sodium fructose diphosphate, 0.5g of sodium bisulfite, 25g of mannitol, and 600ml of water for injection.
[0023] The preparation method is as follows:
[0024] Stir and dissolve sodium fructose diphosphate, sodium bisulfite, and mannitol in sequence with water for injection, add 0.3% activated carbon by weight of the solution, stir for 20 minutes ...
Embodiment 2
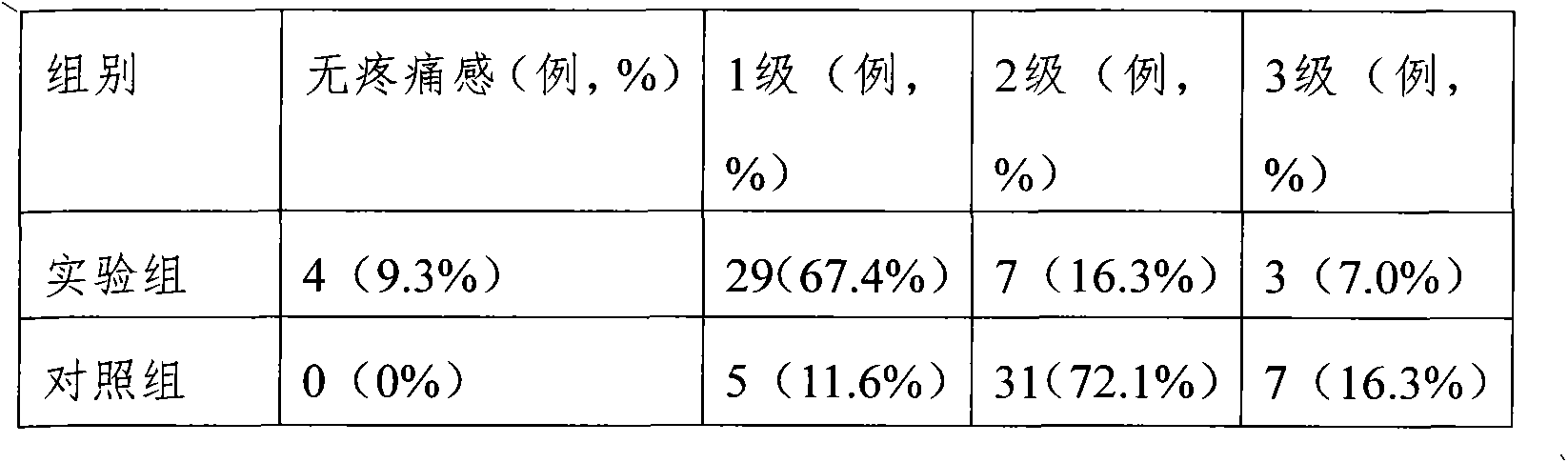
[0026] 1. Vascular stimulation experiment
[0027] Samples: Experimental groups 1-5 are Fructose Diphosphate Sodium Injection formulated in Formulas 1-5 of Example 1, and the negative control group is 0.9% Sodium Chloride Injection.
[0028] Test method: Japanese big-eared white rabbits, weighing 2-2.5kg, were raised in the animal room for one week before the experiment to adapt to the environment. Five rabbits were used, and the experimental group was injected with 5 mL / kg in the right ear vein of the rabbits, and the negative control with the same injection amount was injected into the left ear vein. Use a No. 6 scalp needle to inject into the ear vein. The direction of the needle point to puncture the blood vessel is from the distal end to the proximal end, and the administration speed is 2-3mL / min. Dosing once a day for 7 consecutive days. The animals and injection sites were observed visually before administration and 48 hours after the last administration, and histopat...
Embodiment 3
[0037] Embodiment 3 stability test
[0038] The sample of embodiment 1 configuration 1~5 gained and the sample of contrast 1 (in embodiment 1 configuration 2 do not add mannitol), according to the requirement of national drug standard WS1-(X-063)-2001Z, carry out appearance, clarity, relevant Substance (free phosphate) and content detection and long-term stability test.
[0039] Take the samples of configurations 1 to 5 in Experimental Example 1, place them for 6 months at a temperature of 40°C ± 2°C, and a relative humidity of 75% ± 5%, and conduct an accelerated test to determine the content of active ingredients. The results are shown in Table 1.
[0040] Table 2 Accelerated test results
[0041]
[0042] Through six months of accelerated observation at constant temperature, the related substances and contents of the sodium fructose diphosphate injections in configurations 1 to 5 of Example 1 were all qualified, and all other inspections were in compliance with the regu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com