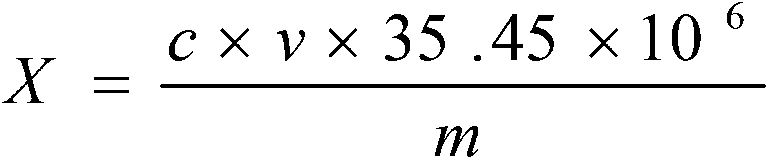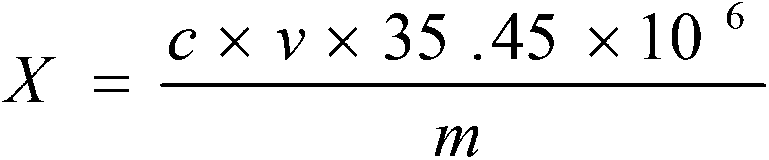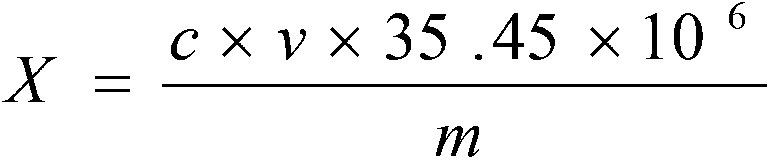Detection method for chlorine ion content in lithium-ion battery electrolyte
A technology of chloride ion content and lithium ion battery, applied in the field of chemical analysis, can solve the problems of large method error, high detection limit, unsatisfactory repeatability and reproducibility, etc., and achieves simple and fast operation, low detection limit and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A. Preparation of 0.1mol / L silver nitrate standard solution:
[0039] Weigh 17.5g of silver nitrate, dissolve in 1000mL of ethanol, and shake well. The solution is stored in a brown bottle.
[0040] B. Calibration with 0.1mol / L silver nitrate standard solution:
[0041] Weigh 0.22g of the reference reagent sodium chloride, and use a potentiometric titrator (the silver electrode is the indicator electrode, and the glass electrode is the reference electrode) for calibration.
[0042] D. Preparation of 0.001mol / L silver nitrate standard solution:
[0043] The prepared and calibrated 0.1mol / L silver nitrate standard solution was accurately diluted 100 times with ethanol.
[0044] E. Detection of chloride ions in the electrolyte:
[0045] Accurately weigh about 60g of lithium-ion battery electrolyte sample (accurate to 0.01g) into the titration cup, and after stirring, titrate the solution with standard silver nitrate with a concentration of 0.001mol / lL, and use Metrohm ...
Embodiment 2
[0059] With reference to the experimental procedure of Example 1, the solvent ethanol of the silver nitrate standard solution was replaced with acetone, and the other steps were the same.
[0060] Sample inspection results:
[0061]
[0062] Recovery test results:
[0063]
Embodiment 3
[0065] With reference to the experimental procedure of Example 1, the solvent ethanol of the silver nitrate standard solution was replaced with ethyl acetate, and the other steps were the same.
[0066] Sample inspection results:
[0067]
[0068] Recovery test results:
[0069]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com