Preparation method of supported ultrafine amorphous metal nickel catalyst for realizing catalytic full hydrogenation of aromatic ring
A technology of amorphous metal and nickel catalyst, which is applied in the field of preparation of catalyzed full hydrogenation of aromatic ring supported ultrafine amorphous metal nickel catalyst, can solve the problem of low volatilization temperature, achieve complete hydrogenation, and easy preparation method and technology , the effect of mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Supported ultrafine amorphous metal nickel catalyst that catalyzes full hydrogenation of aromatic rings:
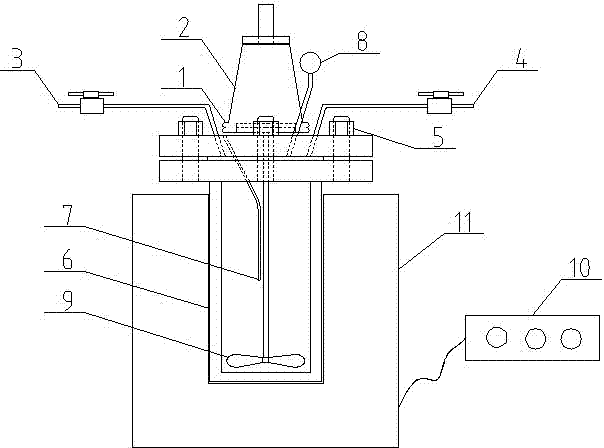
[0026]Add 40 ml of petroleum ether and the β-Al 2 o 3 Carrier 4g, nickel carbonyl Ni(CO) 4 After 100 milliliters, cover the autoclave still body 6, and fix and seal by bolt 5. Open the valves of the gas inlet 3 and the gas outlet 4 of the reactor, and slowly feed high-purity nitrogen in the reactor for 0.1 hour to replace the air in the original reactor. Repeat the same operation three times for nitrogen replacement, open the gas inlet 3 valve of the reaction kettle and close the gas outlet 4 valve, and slowly introduce high-purity hydrogen from the gas inlet 3 to the pressure gauge 8 on the autoclave. After the pressure is 8MPa, close the air inlet 3 valve.
[0027] Under the hydrogen atmosphere, by adjusting the parameters of the control panel 10, the motor 1 is turned on and the magnetic stirrer 2 is driven to slowly stir the mixture of organic solven...
Embodiment 2
[0037] 1. Supported ultrafine amorphous metal nickel catalyst that catalyzes full hydrogenation of aromatic rings:
[0038] Add 200 ml of diethyl ether, 10 g of ZSM-5 zeolite carrier of the catalyst, nickel carbonyl Ni(CO) 4 After 60 milliliters, cover the inside of the autoclave kettle body 6, and fix and seal it by bolts 5. Open the valves of the gas inlet 3 and the gas outlet 4 of the reactor, and slowly feed high-purity nitrogen in the reactor for 0.1 hour to replace the air in the original reactor. Repeat the same operation three times for nitrogen replacement, open the gas inlet 3 valve of the reaction kettle and close the gas outlet 4 valve, and slowly introduce high-purity hydrogen from the gas inlet 3 to the pressure gauge 8 on the autoclave. pressed for 5MPa Finally close the air inlet 3 valve.
[0039] Under the hydrogen atmosphere, by adjusting the parameters of the control panel 10, turn on the motor 1 and drive the magnetic stirrer 2, slowly stir the mixt...
Embodiment 3
[0047] 1. Supported ultrafine amorphous metal nickel catalyst that catalyzes full hydrogenation of aromatic rings:
[0048] In the autoclave with the lid opened, 100 ml of methanol was added successively, and the catalyst TiO 2 Zeolite carrier 20g, nickel carbonyl Ni(CO) 40 Cover the inside of the autoclave body 6 with 10 milliliters, and fix and seal it with bolts 5 . Open the valves of the gas inlet 3 and the gas outlet 4 of the reaction kettle, and slowly feed high-purity nitrogen in the kettle body for 1 hour to replace the air in the original kettle. Repeat the same operation three times for nitrogen replacement, open the gas inlet 3 of the reaction kettle and close the valve of the gas outlet 4, and slowly introduce high-purity hydrogen from the gas inlet 3 until the pressure gauge 8 on the autoclave shows the initial pressure When it is 2MPa, the air inlet 3 is closed until it is closed.
[0049] Under the hydrogen atmosphere, by adjusting the parameters of the c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com