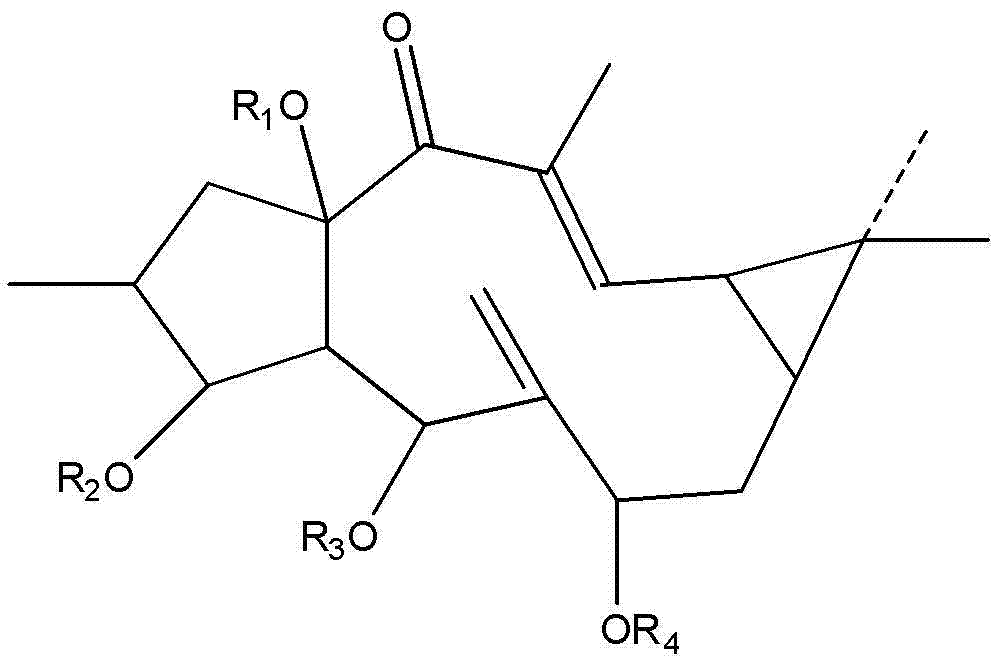
Euphorbia lathyris large ring diterpenoid compound and application thereof
A technology of diterpenoids and compounds, applied in the field of macrocyclic diterpenoids of capers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
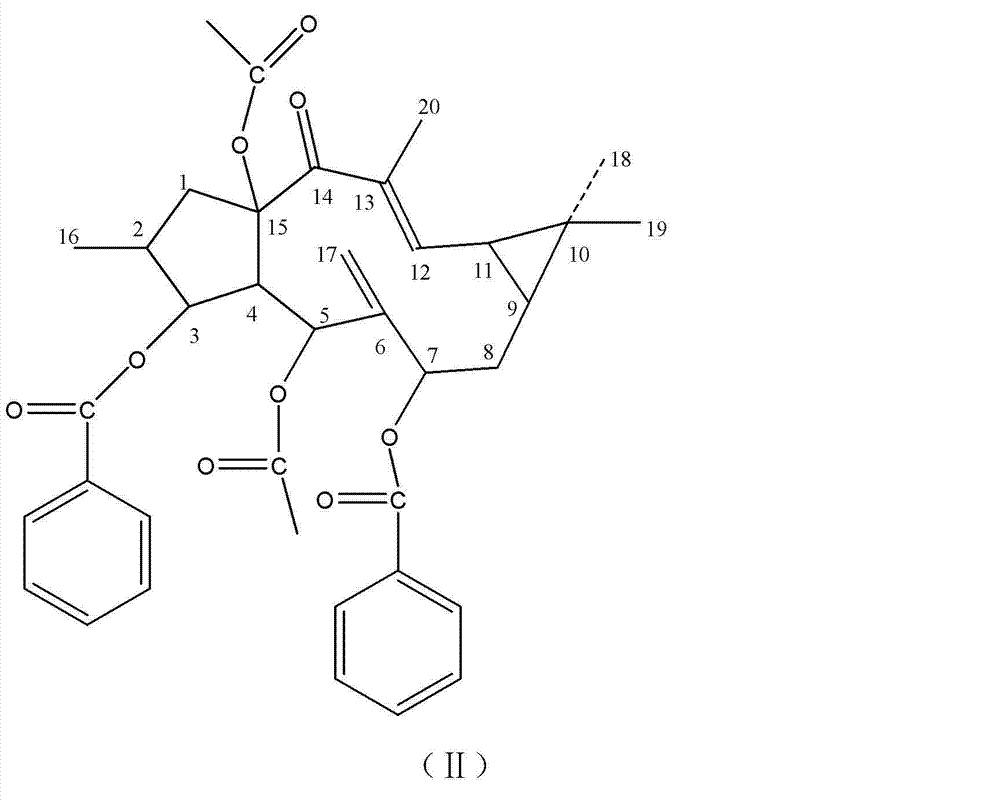
[0026] Take 2000g of caperia sativa fruit, grind it, and extract it twice with 5000 ml of ethanol at 80°C under reflux, each time for 3 hours, to obtain an ethanol extract; recover ethanol from the ethanol extract under reduced pressure to obtain an ethanol extract. Disperse the ethanol extract in 3000ml of water, extract twice with 3000ml of petroleum ether to obtain a petroleum ether extract, concentrate the petroleum ether extract under reduced pressure, recover petroleum ether, and obtain a petroleum ether extract. The petroleum ether extract was chromatographed on a silica gel column and eluted with a gradient of petroleum ether-acetone in different proportions. Crystals were precipitated in the eluent with a ratio of petroleum ether:acetone of 5:1, and a large number of crystals were precipitated after concentration. According to the NMR test, the crystal is the compound shown in the above formula (II), and the molecular formula is C 38 h 42 o 9 , colorless, its NMR da...
Embodiment 2
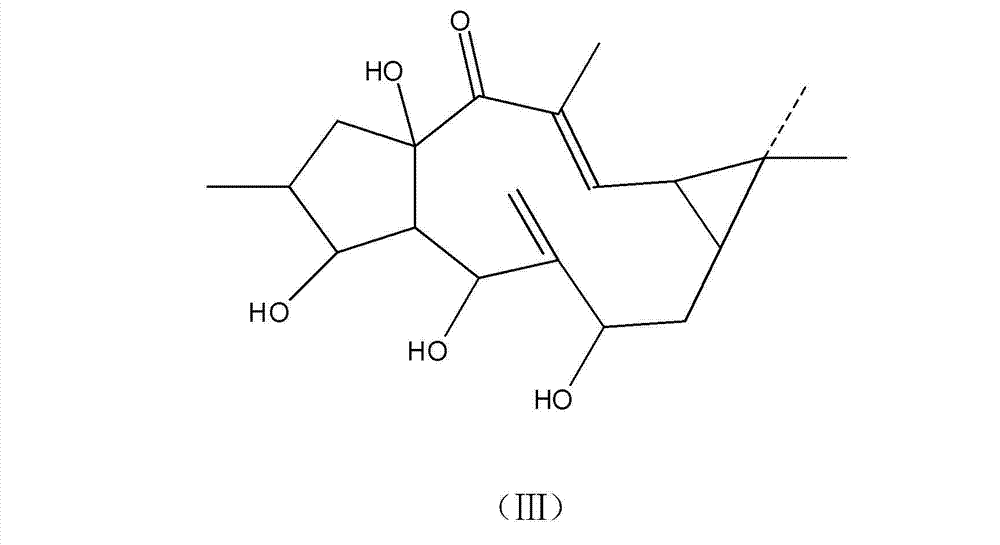
[0034] 1000 mg of the compound prepared in Example 1 was dissolved in 100 ml of methanol solution saturated with sodium hydroxide, and refluxed in a water bath at 60° C. for 12 hours. Concentrate under reduced pressure to remove methanol, add ethyl acetate to dissolve, then wash away water-soluble substances such as sodium hydroxide with water to obtain ethyl acetate liquid, concentrate the ethyl acetate liquid to 10ml, leave it for a period of time, until crystals are precipitated, and filter to obtain crystals , the crystal was tested by proton nuclear magnetic spectrum, showing that there were no acetate groups and benzoate groups, and then tested by mass spectrometry, its molecular formula was C 20 h 30 o 5 , the structural formula is shown in formula (Ⅲ).
Embodiment 3
[0036] Anticancer activity test of the compound of embodiment 1
[0037] The inhibitory effect of compounds on cells was determined by XTT method.
[0038] Prepare XTT into a 1g / L solution with serum-free RPMI-1640 medium, and filter to sterilize with a 0.22 μm filter membrane. Preparation of PMS: PBS (containing 8.0 grams of sodium chloride per liter, 0.20 grams of potassium chloride, 1.44 grams of sodium dihydrogen phosphate, 0.24 grams of potassium dihydrogen phosphate, pH 7.4) was used to prepare a 5 mmol / L PMS stock solution, 0.22 Sterilize with a μm filter membrane and store in the dark at 4°C. Add 50μL of PMS to every 10mL of newly prepared XTT (1g / L), and mix well to get the XTT-PMS use solution, which is ready for immediate use.
[0039] Determination method: take SMMC-7221, L in the logarithmic growth phase 342 , MCc80-3 cells were blown into a single cell suspension, and counted by trypan blue staining (the percentage of viable cells > 95%), and the cells were co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com