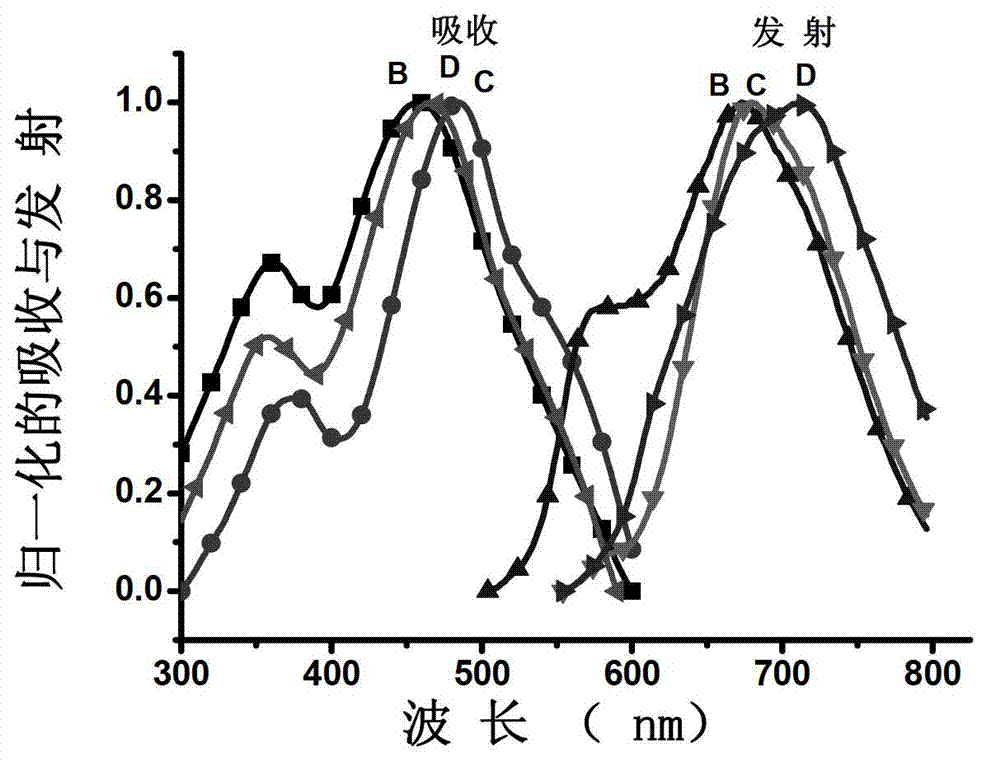
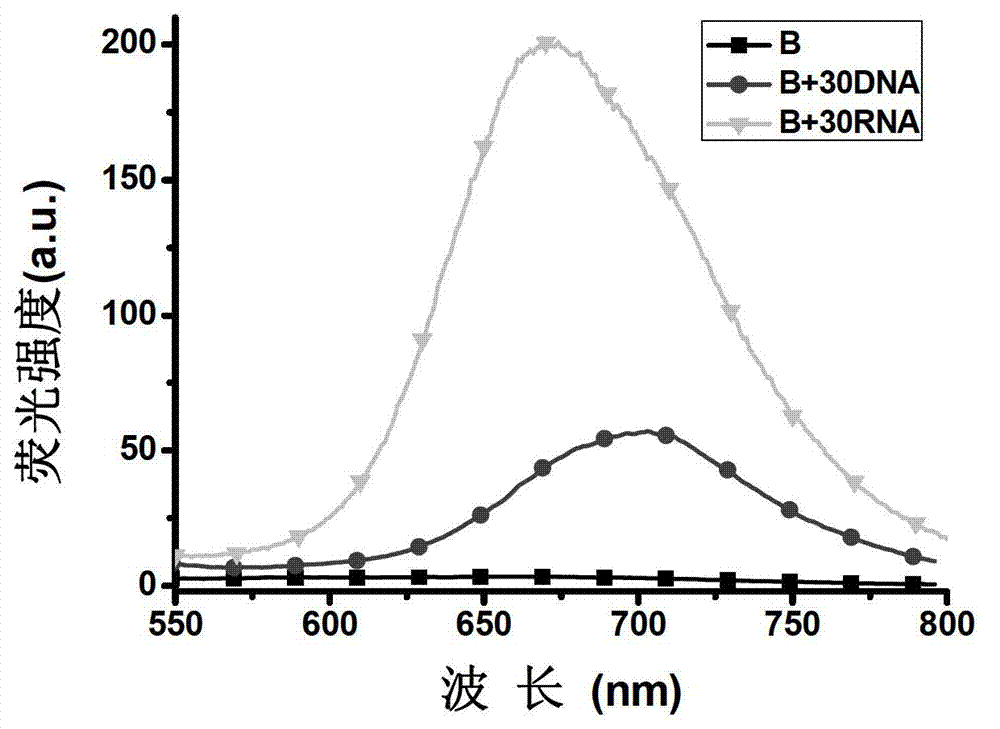
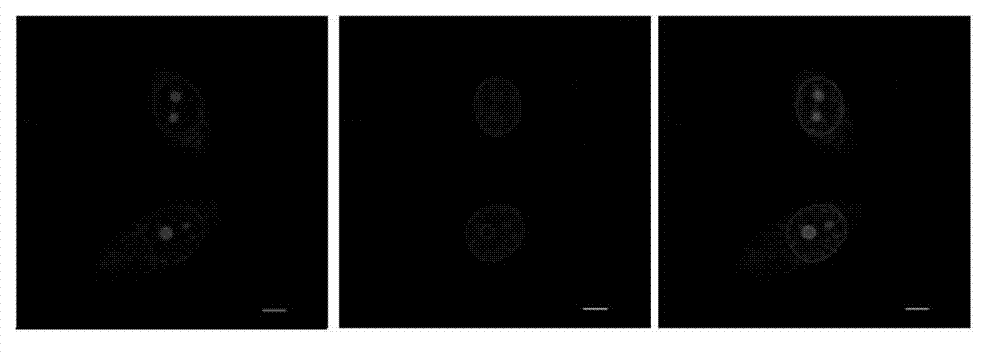
Half cyanine dye compound, preparation method and application of half cyanine dye compound
A technology of cyanine dyes and compounds, which is applied in the field of fine chemicals, can solve the problems that dyes are difficult to enter cells (such as requiring 24 hours of incubation, cannot enter the nucleus, etc.), and achieve the effects of avoiding fluorescence background interference, simple structure, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of new compound intermediate N-methyl-2-methylbenzothiazole quaternary ammonium salt (compound 1):
[0047]
[0048] Add 20mmol of 2-methylbenzothiazole and 40mmol of methyl iodide into a 100ml round bottom flask containing 20ml of toluene under argon protection. The reaction was heated and refluxed for 24 hours and then stopped. The heating was based on the fact that the reaction system began to reflux, and the temperature was about 110 degrees. After the mixture was cooled, the precipitate was filtered and the filter cake was washed with ether. After drying, an off-white solid powder was obtained with a crude yield of 85%.
Embodiment 2
[0050] Preparation of Compound A:
[0051]
[0052] (1) Synthesis of intermediate 4-dimethylamino-1,3-benzaldehyde (compound A)
[0053] 50 g (326 mmol) POCl 3 It was added dropwise to 81.2 g (1.11 mmol) of DMF to obtain a pale yellow solution. Add the obtained solution dropwise to 12.1g (100mmol) N,N-dimethylaniline solution under ice bath condition. After the dropwise addition, move the solution into an oil pan and react at 80-90°C for 10h. After the reaction, the reaction solution was poured into a mixture of 600 g of ice and 150 mL of 38% NaOH and stirred to obtain a brown precipitate, which was filtered and the obtained mixture was separated by a silica gel column. Compound A (4.5g) was obtained by washing with petroleum ether: ethyl acetate (5:1-4:1), with a yield of 25%. 1 H NMR (400MHz, CDCl 3 )δ=10.04(s,1H), 9.86(s,1H), 7.92(d,J=8.8,1H), 7.03(d,J=8.8,1H).
Embodiment 3
[0055] Preparation of Compound B:
[0056]
[0057] Add 11 mmol of piperidine to an ethanol solution of 3.3 g (11.0 mmol) 1,2,3,3-tetramethylindole quaternary ammonium salt and stir for 30 min, then add 890 mg (5.0 mmol) of compound A to an ethanol solution for 1 h Dropped into the refluxing above-mentioned mixed solution, the reaction continued for 6 hours, a dark brown precipitate was produced and filtered, and recrystallized in ethanol to obtain 1.5 g of the product, with a yield of 40.4%. 1 H NMR (400MHz, CDCl 3 )δ=9.53(d,J=1.9,1H),8.72(d,J=16.0,1H),8.60(dd,J=9.0,1.9,1H),8.24(d,J=16.0,1H),8.14 (d,J=16.0,1H),7.96(d,J=16.0,1H),7.70–7.43(m,8H),7.28(s,1H),7.17(d,J=9.0,1H),4.56( s,3H),4.44(s,3H),3.18(s,6H),1.97(s,6H),1.89(s,6H). 13 C NMR (100MHz,D 2 O)δ=182.3, 182.2, 159.1, 154.7, 150.9, 143.4, 143.2, 141.4, 137.0, 136.6, 129.8, 129.6, 129.3, 129.2, 126.7, 124.3, 122.8, 122.71, 118.2, 1133.8, 5, 1 52.4, 45.4, 37.5, 36.9, 27.4, 27.2. HRMS (TOF-MS EI + ) calculated for C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com