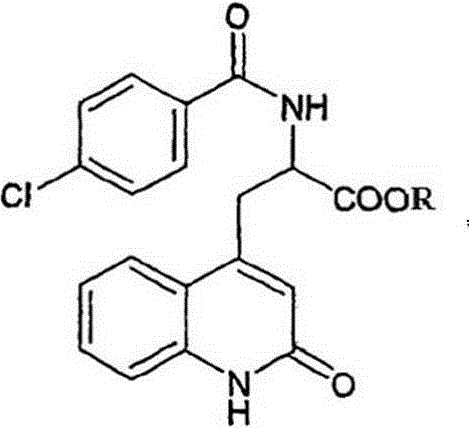
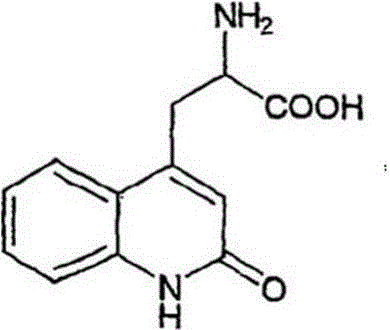
The synthetic method of rebamipide
A synthesis method and technology of rebamipide, applied in the field of rebamipide synthesis, can solve the problems of high production cost, low content of rebamipide, easy generation of impurities, etc., achieve less impurities, reduce production cost, improve yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1, in a 500mL beaker, add 14g of raw amino acid salt, 2-amino-3-(1.2-dihydro-2-oxo-4-quinolyl) propionate hydrochloride to 250mL of purified water, add hydrogen Sodium oxide 5g, increase the temperature to 55°C, add 1g of activated carbon, stir to dissolve and filter, take the filtrate, cool down to 0°C, add dropwise the mixture of 4-chlorobenzoyl chloride and acetone or N,N-dimethylformamide Mixed solution, wherein the mixed solution containing 9.6g of 4-chlorobenzoyl chloride was stirred and added dropwise for reaction for 2 hours, left to stand for 2 hours after the reaction was completed, and the transition body was obtained by suction filtration, and the transition body was dissolved in 200mL purified water, and the temperature was controlled at At 20°C, add acid dropwise until the pH is 2, stop the dropwise addition and continue to stir for 2 hours, filter with suction to get a cake-like solid, wash it repeatedly with 70°C purified water and ethanol until t...
Embodiment 2
[0021] Example 2, in a 3000mL beaker, add 70g of raw amino acid salt, 2-amino-3-(1.2-dihydro-2-oxo-4-quinolyl) propionate hydrochloride to 250mL of purified water, add hydrogen Sodium oxide 25g, increase the temperature to 65°C, add 5g of activated carbon, stir to dissolve and filter, take the filtrate, cool down to 2°C, add dropwise the mixture of 4-chlorobenzoyl chloride and acetone or N,N-dimethylformamide The mixed solution, the mixed solution containing 48g of 4-chlorobenzoyl chloride, was stirred and added dropwise for 2 hours. After the reaction was completed, it was left to stand for 2 hours, and the transition body was obtained by suction filtration. The transition body was dissolved in 1000mL purified water, and the temperature was controlled at 20 ℃, add acid dropwise until the pH is 2, stop the dropwise addition and continue to stir for 2 hours, filter with suction to obtain a cake-like solid, wash it repeatedly with 70°C purified water and ethanol until the pH is 7...
Embodiment 3
[0022] Example 3, 14kg raw material amino acid salt, 2-amino-3-(1.2-dihydro-2-oxo-4-quinolyl) propionate hydrochloride was added to 250L purified water in a 300L glass-lined reaction tank , add 5kg of sodium hydroxide, increase the temperature to 70°C, add 1kg of activated carbon, stir to dissolve and filter, take the filtrate, cool down to 5°C, add dropwise 4-chlorobenzoyl chloride / acetone or N,N-dimethyl The mixed solution of formamide, wherein containing the mixed solution of 9.6kg of 4-chlorobenzoyl chloride, stirred and added dropwise to react for 2 hours, left standstill for 2 hours after the reaction was finished, and obtained the transition body by suction filtration, and the transition body was dissolved in 200l purified water, Control the temperature at 20°C, add acid dropwise until the pH is 2, continue stirring for 2 hours after stopping the dropwise addition, filter with suction to obtain a cake-like solid, rinse repeatedly with 70°C purified water and ethanol unti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com