Recombined pichia pastoris strain for commonly expressing glucamylase and alpha-amylase and construction method thereof as well as mixed enzyme preparation
A technology of glucoamylase and Pichia pastoris, applied in the biological field, can solve the problem of no simultaneous expression of α-amylase, and achieve the effects of reducing reverse reactions, improving hydrolysis efficiency, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1. Construction and transformation of recombinant expression vector pPIC9KGla
[0034] 1.1 Primer design
[0035] R.pGAf (SEQ ID NO.3): ATGCGTTATGCAACCCCGC
[0036] R.pGTr (SEQ ID NO.4): GGCGTTATTTATTACCCTCTTTTGACC
[0037] R.pGEf (SEQ ID NO.5): G GAATTC ATGCGTTATGCAACCCCGC
[0038] R.pGNr (SEQ ID NO.6): TT GCGGCCGC TTACCCTCTTTTGACCA
[0039] 1.2 Amplification of cDNA sequence of Mucor pusillus glucoamylase
[0040] Using Rhizomucor pusillus (purchased from the General Microbiology Center of the Chinese Microbial Culture Collection and Management Committee in Beijing, CGMCC, strain number 3.204) cDNA first strand as a template, PCR amplification was carried out with R.pGAf / R.pGTr primers , Cloned the glucoamylase (Gla) gene, its sequence is shown in SEQ ID NO.1. PCR reaction conditions: 94°C for 5min; 94°C for 30s, 60°C for 30s, 72°C for 2min, 20 cycles (annealing temperature decreased by 0.5°C for each cycle); 94°C for 30s, 54°C for 30s, 72°C for 2min, 15 cycles; 72℃...
Embodiment 2
[0055] Example 2. Construction and transformation of recombinant expression vector pPICZαAmy
[0056] 2.1 Primer design
[0057] R.pA-f (SEQ ID NO.7): ATGAAATTCAGCATCTCTCTCTCGG
[0058] R.pA-r (SEQ ID NO.8): TTAAGCAGAGGTGAAGATAGCGGA
[0059] R.pAEf (SEQ ID NO.9): GAATTC AGCCCTTTGCCCCAACAGCA
[0060] R.pANr (SEQ ID NO.10): TT GCGGCCGC TTAAGCAGAGGTGAAGATAG
[0061] 2.2 Amplification of Mucor pusillus α-amylase
[0062] Using the first strand cDNA of Mucor pusillus as a template, PCR amplification was carried out with primers R.pA-f / R.pA-r, and the α-amylase (Amy) gene was cloned. Its sequence is shown in SEQ ID NO.2. . PCR reaction conditions: 94°C for 5min; 94°C for 30s, 65°C for 30s, 72°C for 1min30s, 30 cycles (annealing temperature decreased by 0.5°C for each cycle); 94°C for 30s, 50°C for 30s, 72°C for 1min30s, 10 cycles; 72℃10min, store at 4℃. The PCR products were subjected to agarose gel electrophoresis, recovered and connected to pMD19-T Simple Vector, and transformed into E...
Embodiment 3
[0071] Example 3. Verification of amylase activity produced by recombinant Pichia pastoris transformants
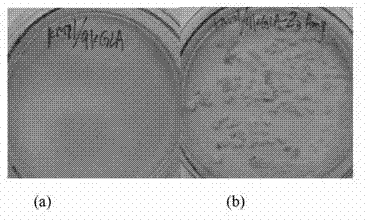
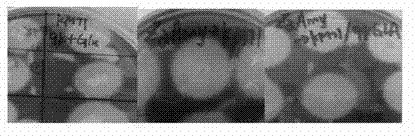
[0072] (1) Pick the recombinant Pichia strains KM71 / 9KGla, KM71 / ZαAmy, KM71 / 9KGla-ZαAmy, and inoculate a single colony of BMMY plates containing 2% soluble starch. Place them in an incubator at 30°C, and grow them on the plate every 24h. Add 200ul filtered and sterilized absolute ethanol to the lower layer;
[0073] (2) Develop the color with iodine solution after 2-3 days, observe the size of the hydrolysis circle around the transformant, and judge whether the transformant successfully expresses Mucor pusillus amylase.
[0074] The result is figure 2 As shown, hydrolysis circles appear around the recombinant Pichia pastoris transformants, indicating that the recombinant Pichia pastoris strains KM71 / 9KGla, KM71 / ZαAmy, and KM71 / 9KGla-ZαAmy can secrete and express amylase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com