Method for preparing lithium ion sieve MnO2.0.5H2O and precursor thereof Li1.6Mn1.6O4
A li1.6mn1.6o4, ion sieve technology, applied in chemical instruments and methods, other chemical processes, inorganic chemistry, etc., can solve the problems of complex synthesis of MnOOH, instability of MnOOH, high operating conditions, etc., and it is easy to achieve product ratio Controlled, stable properties, wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] Manganese series ion sieve adsorbent MnO of the present invention 2 0.5H 2 The preparation method of O and precursor thereof, comprises the steps:
[0032] (1) Add 0.1-0.3mol / L potassium permanganate solution and 3.0-5.0mol / L lithium hydroxide solution into 2-4mol / L manganese chloride solution at a flow rate of 3-10mL / min while stirring , wherein the molar ratio of manganese chloride to potassium permanganate is (3.8-4.5):1, and the molar ratio of lithium hydroxide to potassium permanganate is (12-25):1;
[0033] (2) Transfer the brown solution obtained in step (1) to a 1L polytetrafluoroethylene hydrothermal kettle, and conduct a hydrothermal reaction at 120-200°C for 6-24 hours to obtain the intermediate product LiMnO 2 ;
[0034] (3) LiMnO 2 After washing with water and suction filtration, dry at 60-100°C for 3-6 hours, and then calcinate at 300-500°C for 4-48 hours in an oxidizing atmosphere (air atmosphere or oxygen atmosphere) to obtain a lithium adsorbent pr...
Embodiment 1
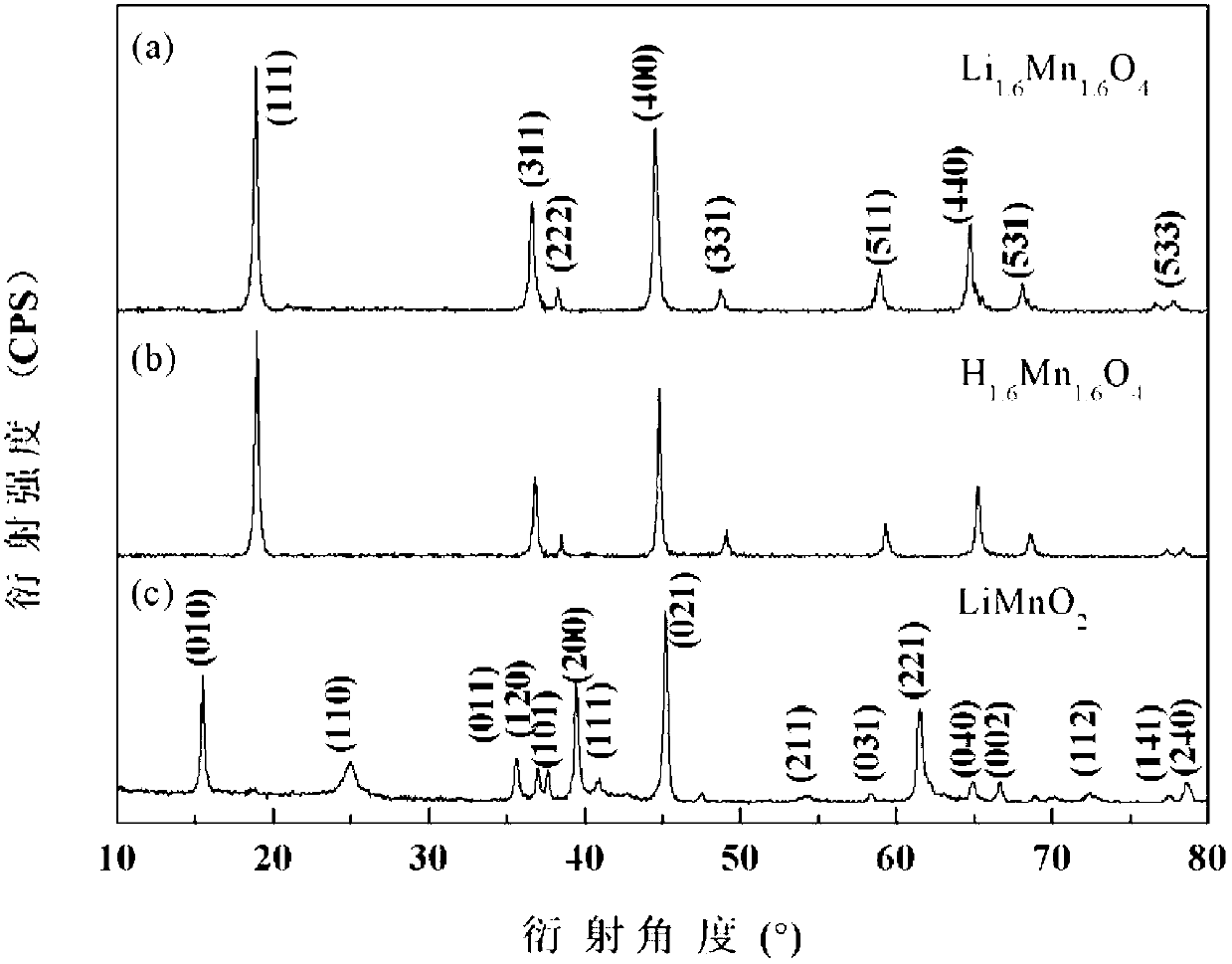
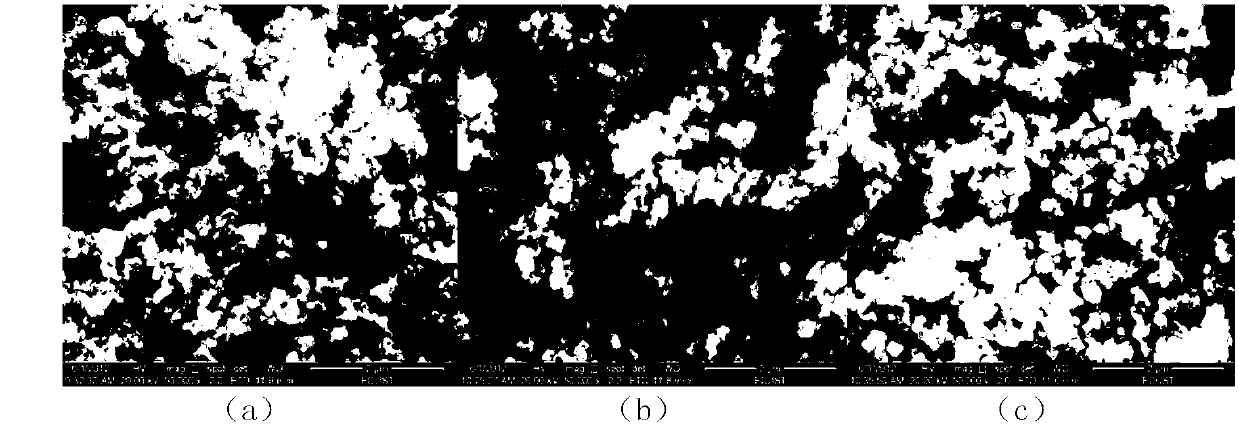
[0039] Add 0.1mol / L potassium permanganate solution and 5.0mol / L lithium hydroxide solution to 2mol / L manganese chloride solution at a flow rate of 3mL / min under vigorous stirring, wherein the added manganese chloride and permanganese The molar ratio of potassium permanganate is 4:1, and the molar ratio of lithium hydroxide and potassium permanganate is 25:1; the obtained brown solution is hydrothermally reacted at 180°C for 6 hours in a 1L polytetrafluoroethylene hydrothermal kettle , to obtain the intermediate product LiMnO 2 , the XRD pattern of the product is shown in figure 1 (a), SEM image see figure 2 (a); by figure 1 (a), 2(a), it can be seen that the pure phase LiMnO can be obtained under the hydrothermal conditions adopted 2 , and the particle size of the product is relatively uniform, and the particle size is about 100nm. LiMnO 2 After washing with 30 times the volume of deionized water, suction filtration, drying at 80°C for 6 hours, and calcination at 450°C ...
Embodiment 2
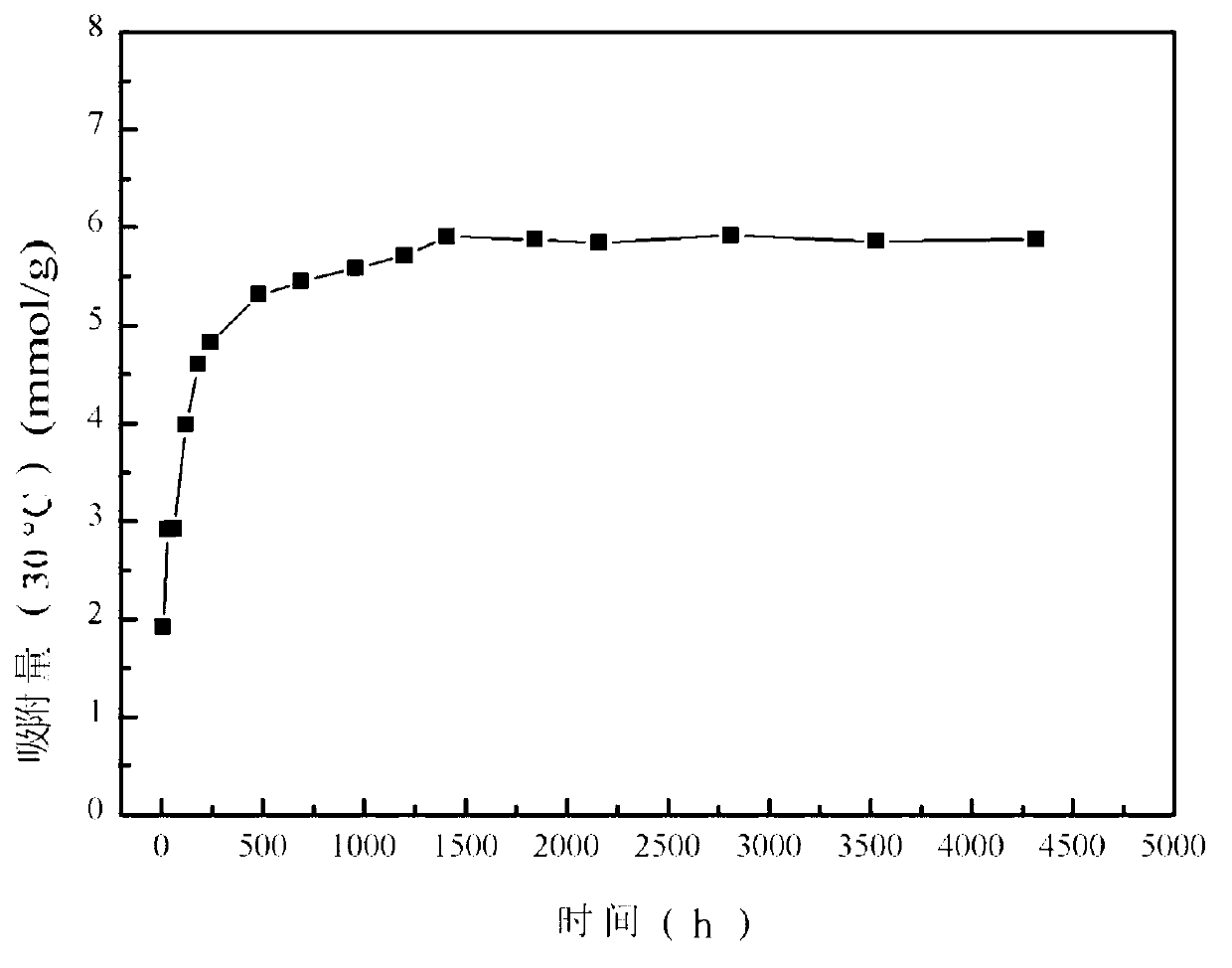
[0042] Add 0.1mol / L potassium permanganate solution and 5.0mol / L lithium hydroxide solution into 2mol / L manganese chloride solution at a flow rate of 3mL / min under vigorous stirring, wherein the manganese chloride and potassium permanganate The molar ratio was 4.5:1, and the molar ratio between lithium hydroxide and potassium permanganate was 16.5:1; the obtained brown solution was transferred to a 1L polytetrafluoroethylene hydrothermal kettle, and hydrothermally reacted at 160°C for 12 hours to obtain Intermediate product LiMnO 2 ; the LiMnO 2 After washing with 30 times the volume of deionized water, suction filtration, drying at 80°C for 6 hours, and calcination at 350°C in air atmosphere for 24h, the lithium adsorbent precursor Li 1.6 mn 1.6 o 4 . Take the precursor 0.8g Li 1.6 mn 1.6 o 4 Add 200mL of 0.1mol / L HCl solution, put it into a constant temperature water bath shaker to oscillate at a frequency of 130rpm, control the temperature at 30°C, and react for 12h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com