Composite catalyst for catalytically oxidizing nitrogen oxide and preparation method of composite catalyst
A composite catalyst, nitrogen oxide technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problems of limited application and complex catalyst preparation process, etc. To achieve the effect of simple and easy preparation process, good application prospect and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of catalyst carrier in the present embodiment:
[0033] Add 0.5g of scallop powder to 50g of zirconia (analytical pure) powder, stir well, add 17ml of distilled water, stir evenly to form a paste, dry in an oven at 60°C for 5 hours to obtain a blocky solid, crush the blocky solid Sieve to obtain 20-40 mesh particles, which is the catalyst carrier.
[0034] The load of catalyst in the present embodiment:
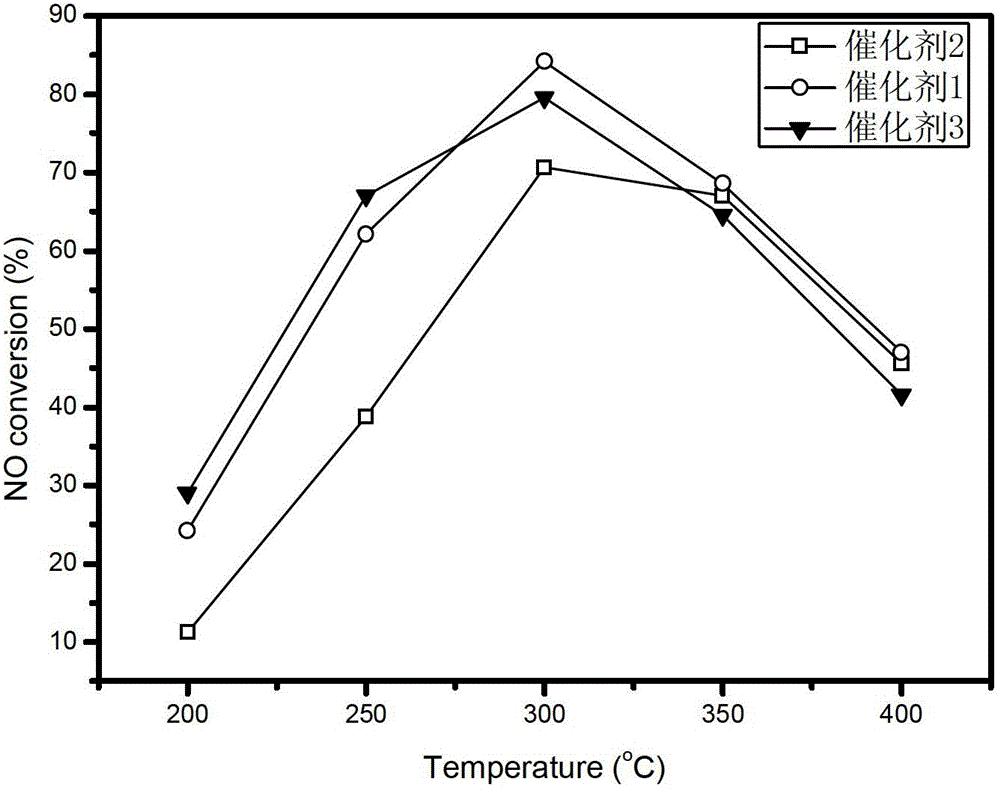
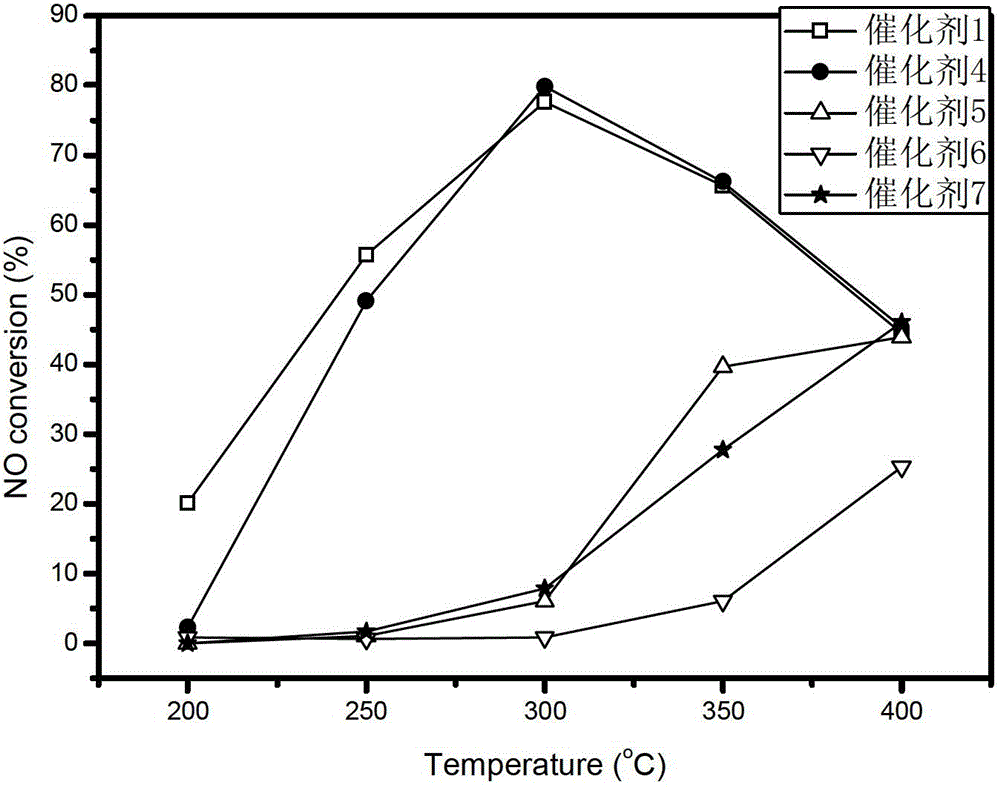
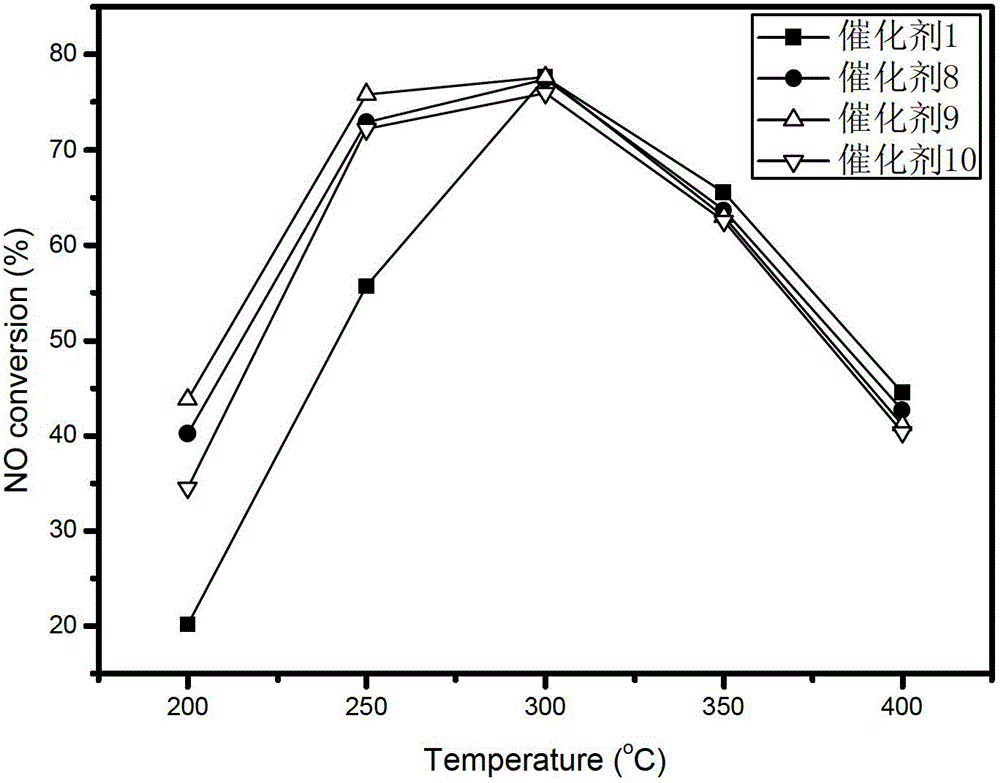
[0035] After taking 3g of the catalyst carrier and adding water by the equal volume impregnation method, the solid-liquid ratio was measured to be 1g: 0.78ml; the Mn(NO 3 ) 2 Solution 3.42ml, containing Mn(NO 3 ) 2 2.60g, diluted with distilled water to 7.80ml, mixed evenly, added to 10g of catalyst carrier, left at room temperature for 24h, then dried at 60°C for 5h, 110°C for 10h, and finally placed in air atmosphere at 450°C for 3h to obtain Mn8 / ZrO 2 The composite catalyst is designated as Catalyst 1.
[0036] Change the volume of the mangan...
Embodiment 2
[0041] The preparation of catalyst carrier in the present embodiment:
[0042] Add 0.5g of scallop powder to 50g of zirconia (analytically pure) powder, stir well, add 17ml of distilled water, stir evenly to form a paste, dry in an oven at 60°C for 5 hours, and obtain a blocky solid, crush the blocky solid Sieve to obtain 20-40 mesh particles, which is the catalyst carrier.
[0043] The loading of catalyst in this embodiment
[0044] Take 3g of catalyst carrier and add water by equal volume impregnation method, and measure its solid-liquid ratio as 1g:0.6ml; weigh 4.242g of cobalt nitrate hexahydrate and add it to 6ml of distilled water, add 10g of catalyst carrier after it dissolves, let stand at room temperature for 24h, then Dry at 60°C for 5h, dry at 110°C for 12h, and finally place in an air atmosphere at 350°C for 3h to prepare Co8.6 / ZrO 2 The composite catalyst is recorded as catalyst 4, and the molar number of metal elements in the active components of catalyst 4 is ...
Embodiment 3
[0050] The preparation of catalyst carrier in the present embodiment:
[0051] Add 0.5g of scallop powder to 50g of zirconia (analytically pure) powder, stir well, add 17ml of distilled water, stir evenly to form a paste, dry in an oven at 60°C for 5 hours, and obtain a blocky solid, crush the blocky solid Sieve to obtain 20-40 mesh particles, which is the catalyst carrier.
[0052] The loading of catalyst in this embodiment
[0053] Take 3g of catalyst carrier and add water by equal volume impregnation method, and measure its solid-liquid ratio as 1g:0.6ml; weigh 0.31g of cerium nitrate hexahydrate and add it to 2.58ml of distilled water, and after it dissolves, add 50% Mn(NO 3 ) 2 Solution 3.42ml, containing Mn(NO 3 ) 2 2.60g, mixed evenly, added to 10g catalyst carrier, left at room temperature for 24h, then dried at 60°C for 5h, 110°C for 12h, and finally placed in air atmosphere at 450°C for 3h to obtain Mn8Ce1 / ZrO 2 The composite catalyst is designated as Catalyst 8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com