Preparation method of diethyl zinc
A technology of diethylzinc and diethylaluminum chloride is applied in chemical instruments and methods, zinc organic compounds, compounds containing elements of Group 3/13 of the periodic table, etc. It can not be too high and the cost is high, so as to achieve the effect of easy industrial production, improve yield and purity, and promote the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
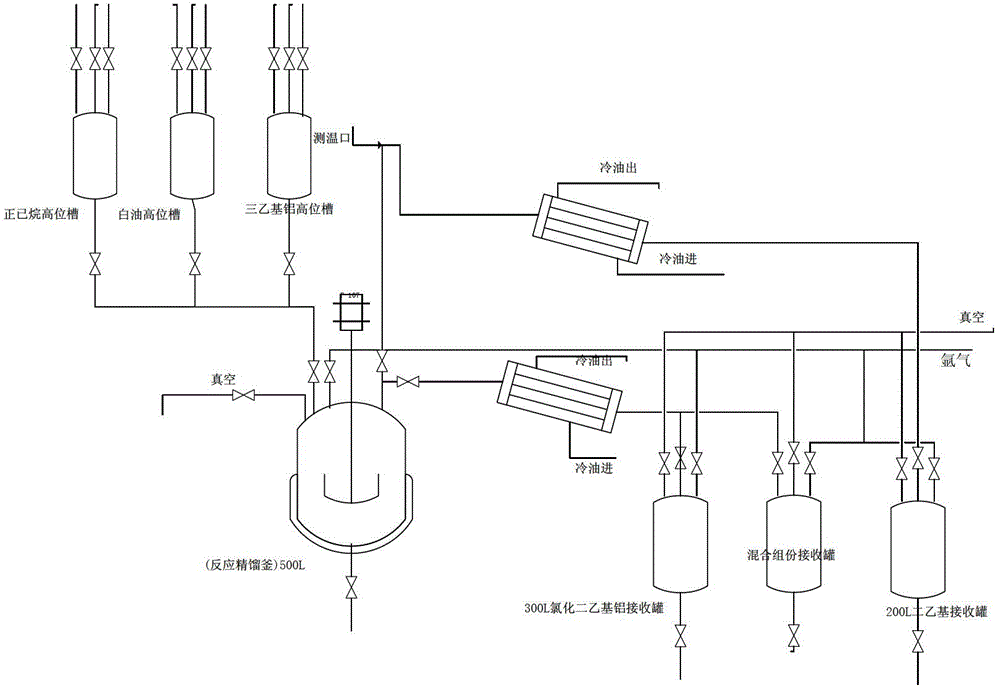
[0030] 1. The reaction kettle, pipeline, high level tank and receiving tank are replaced by argon 2-3 times. Open the manhole, put in 0.74kg of zirconocene dichloride and 170kg of zinc chloride under the protection of argon, heat up to 90°C, stop the argon, dry under reduced pressure for 2-4 hours, cool to below 25°C for later use;
[0031] 2. Under the protection of argon, press the raw material 292.5kg of triethylaluminum into the triethylaluminum high-level tank, and put the high-level tank triethylaluminum under the protection of argon into a slow-stirring reactor. The reactor system Automatically exothermic and slowly rise to 30-35°C, after the addition is complete, heat up to 40-45°C, and react for 2 hours;
[0032] 3. Cool down to below 30°C, open the diethyl zinc distillation valve, distill the diethyl zinc product under reduced pressure (pressure is -0.08-0.09MPa), slowly raise the temperature of the reactor, and collect diethyl zinc with a gas phase temperature of 30...
Embodiment 2
[0038] 1. The reaction kettle, pipeline, high level tank and receiving tank are replaced by argon 2-3 times. Open the manhole, put in 0.59kg of zirconium chloride and 170kg of zinc chloride under the protection of argon, heat up to 90°C, stop the argon, dry under reduced pressure for 2-4 hours, cool to below 25°C for later use;
[0039] 2. Under the protection of argon, press the raw material 292.5kg of triethylaluminum into the triethylaluminum high-level tank, and put the high-level tank triethylaluminum under the protection of argon into a slow-stirring reactor. The reactor system Automatically exothermic and slowly rise to 30-35°C, after the addition is complete, heat up to 40-45°C, and react for 2 hours;
[0040]3. Cool down to below 30°C, open the diethyl zinc distillation valve, distill the diethyl zinc product under reduced pressure (pressure is -0.08-0.09MPa), slowly raise the temperature of the reactor, and collect diethyl zinc with a gas phase temperature of 30-50°C...
Embodiment 3
[0046] 1. The reaction kettle, pipeline, high level tank and receiving tank are replaced by argon 2-3 times. Open the manhole, put in 6.0kg of zirconocene dichloride and 170kg of zinc chloride under the protection of argon, heat up to 90°C, stop the argon, dry under reduced pressure for 2-4 hours, cool to below 25°C for later use;
[0047] 2. Under the protection of argon, press the raw material 292.5kg of triethylaluminum into the triethylaluminum high-level tank, and put the high-level tank triethylaluminum under the protection of argon into a slow-stirring reactor. The reactor system Automatically exothermic and slowly rise to 30-35°C, after the addition is complete, heat up to 40-45°C, and react for 2 hours;
[0048] 3. Cool down to below 30°C, open the diethyl zinc distillation valve, distill the diethyl zinc product under reduced pressure (pressure is -0.08-0.09MPa), slowly raise the temperature of the reactor, and collect diethyl zinc with a gas phase temperature of 30-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com