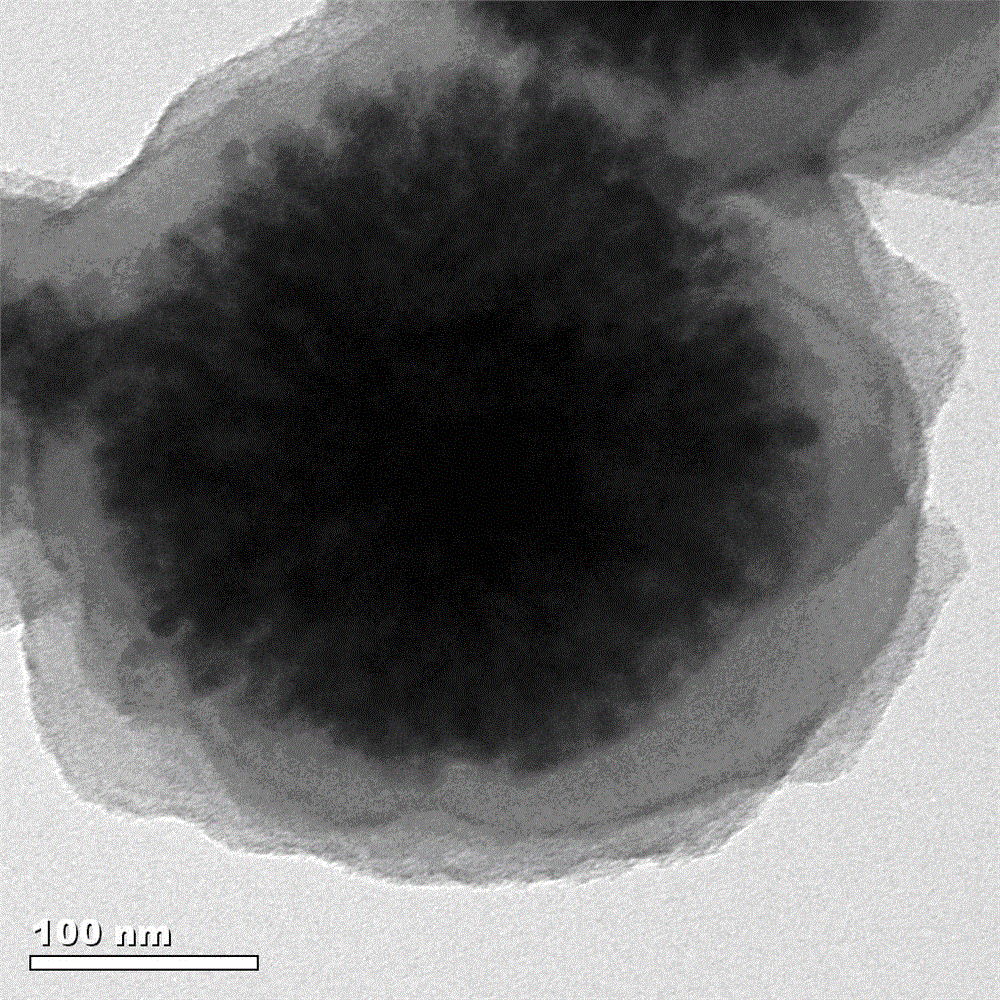
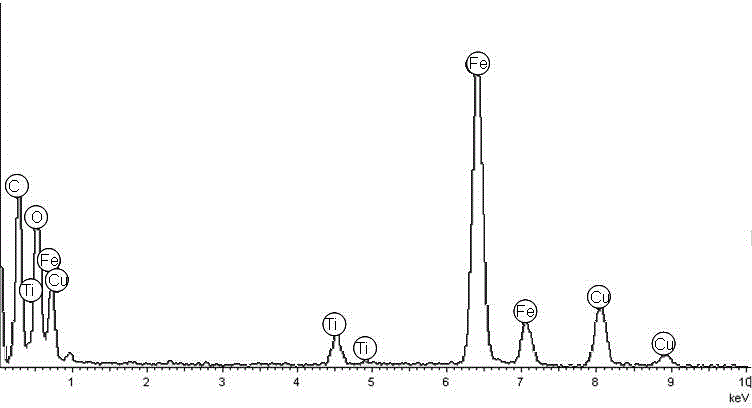
Polydopamine modified magnetic ball, synthetic method of nanomaterial by fixing Ti<4+> on the surface and application thereof
A polydopamine and surface immobilization technology, applied in the direction of material analysis, analysis of materials, and inorganic material magnetism by electromagnetic means, can solve the problems of loss of low-abundance phosphorylated peptides, time-consuming and inconvenient, and achieve good biocompatibility, The effect of simple fixing method and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Polydopamine modified magnetic spheres and immobilized Ti on the surface 4+ Synthesis of Nanomaterials:
[0031] (1) Disperse 10 mg of magnetic spheres into 40 mL of dopamine aqueous solution with a concentration of 1 mg / mL, and make it disperse evenly;
[0032] (2) Under the condition of continuous mechanical stirring, quickly add 10 mL concentration of tris buffered solution of 10 mmol / L (pH is 8.5) to the product obtained in step (1);
[0033] (3) The solution obtained in step (2) was mechanically stirred at room temperature for 10-16 hours;
[0034] (4) washing the product obtained in step (3) three times with deionized water to remove impurities and dopamine monomers on the surface of the product;
[0035] (5) Disperse the product obtained in step (4) into 100 mmol / L of Ti (SO 4 ) 2 solution, mechanical stirring at room temperature for 2-3 hours;
[0036] (6) The product obtained in step (5) was washed three times with deionized water, and dried in a vacuum d...
Embodiment 2
[0039] Polydopamine modified magnetic spheres and immobilized Ti on the surface 4+ Application of Nanomaterials in Enrichment of Phosphorylated Peptides in β-casein:
[0040] (1) Sample preparation: β-casein in 25mmol / L NH 4 HCO 3 Enzymatic hydrolysis at 37 °C for 16 h in the solution.
[0041](2) Enrichment: 10 μL, 2 mg / mL nanomaterials were dispersed into a 200 μL centrifuge tube containing 0.1% (volume ratio) trifluoroacetic acid and 50% (volume ratio) acetonitrile containing the phosphorylated peptide from step (1), Enriched at 37°C for 30 min; washed with a buffer solution of 0.1% (volume ratio) trifluoroacetic acid and 50% (volume ratio) acetonitrile and magnetically separated 3 times; eluted with 5 μL 0.4 M ammonia water for 10 min.
[0042] (3) Mass spectrometry analysis: Take 0.5 μL of the eluate obtained in step (2) to spot the target and perform mass spectrometry analysis. The mass spectrogram is as follows: Image 6 as shown, Figure 8 It is a polydopamine mo...
Embodiment 3
[0044] Polydopamine modified magnetic spheres and immobilized Ti on the surface 4+ Application of Nanomaterials in Enrichment of Phosphopeptides in Mixed Proteins
[0045] (1) Sample preparation: bovine serum albumin (BSA) was reductively alkylated with dithiothreitol and iodoacetamide, and then enzymatically hydrolyzed at 37 °C for 16 h. β-casein in 25mM NH 4 HCO 3 Enzymatic hydrolysis at 37°C for 16 h in the solution. Add bovine serum albumin (BSA) and β-casein at a molar ratio of 500:1 to a centrifuge tube containing 0.1% (volume ratio) trifluoroacetic acid and 50% (volume ratio) acetonitrile.
[0046] (2) Enrichment: 10 μL, 2 mg / mL The nanomaterial obtained in Example 1 was dispersed into 200 μL containing 0.1% (volume ratio) trifluoroacetic acid and 50% (volume ratio) of the phosphorylated peptide in step (1) In an acetonitrile centrifuge tube, enrich at 37°C for 30 min; wash with a buffer solution of 0.1% (volume ratio) trifluoroacetic acid and 50% (volume ratio) ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com