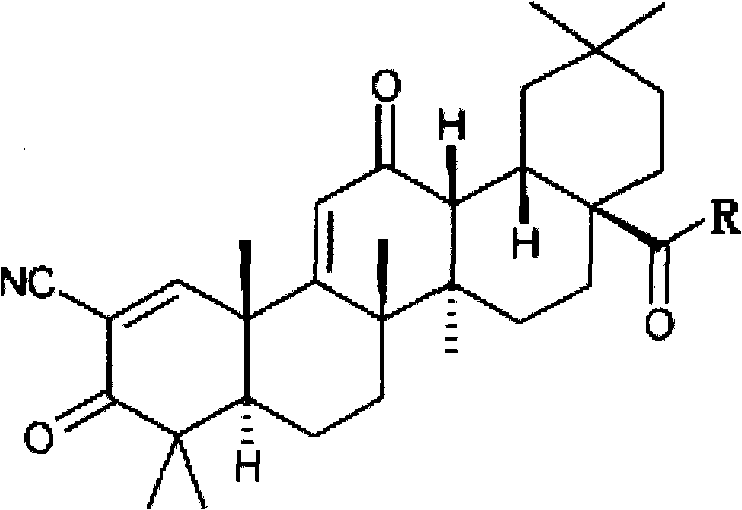
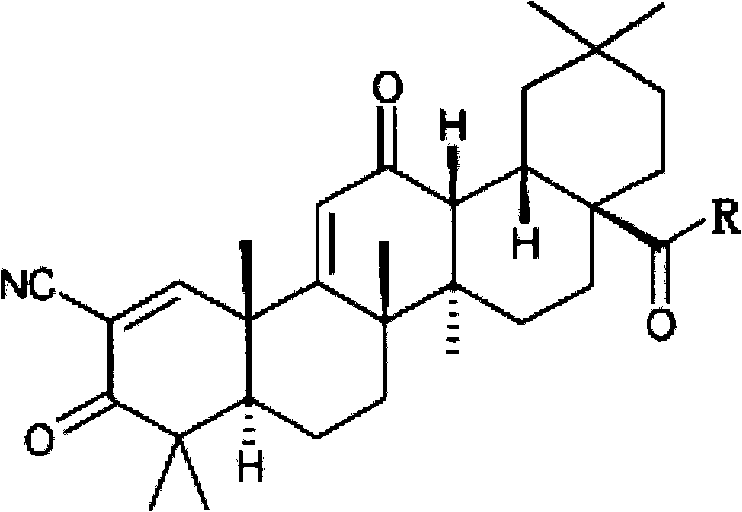
Application of oleanolic acid derivative and pharmaceutically acceptable salts thereof in diabetic eye disease treatment
A technology for diabetic eye disease and zidonolic acid, applied in the field of long-acting polymer preparations, to avoid surgical trauma, improve bioavailability, and long-term effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] ①Mix 8ml acetone and 2ml ethanol;
[0020] ② Take 25mg of polysebacic anhydride (PSA) and add it to 1ml ① to prepare an organic phase;
[0021] ③Add 6mg of CDDO-ME to the organic phase prepared in ②;
[0022] ④ Take propylene glycol block polyether (i.e. F68) and Tween 80 (i.e. T80) (1:1) to make an aqueous solution containing 2% emulsifier, and add dextran-70 (i.e. Dextran-70) to it , adjust the pH of the solution to be 7.8;
[0023] 5. quickly drop the organic phase prepared in step 2 to the external aqueous phase prepared in 4;
[0024] ⑥ Removing the organic solvent to obtain a colloidal solution of nanoparticles.
Embodiment 2
[0026] ①Dissolve 40mg CDDO-IM and 150mg polylactide-glycolide (PLGA) with 1.8ml dichloromethane and 0.2ml DMSO in a 30°C water bath;
[0027] ②Add 1.5% polyvinyl alcohol to 7ml to ①, and ultrasonic;
[0028] ③ Pour ② into low-concentration polyethanol, stir at low speed at room temperature for 2 hours, and wait for the organic solvent to evaporate;
[0029] ④ Collect the obtained nanoparticles after centrifugation at 4°C for 60 minutes;
[0030] ⑤ Wash the product obtained in ④ three times with double distilled water to obtain the drug-loaded nanoparticles.
Embodiment 3
[0032] ①Mix 8ml of dichloromethane and 2ml of ethyl acetate;
[0033] ② Take 100mg PLGA and add it to 1ml ① to prepare an organic phase;
[0034] ③Add 20mg CDDO-MA to the organic phase prepared in ②;
[0035] ④ Take F68 and T80 (1:1) to make a 4% aqueous solution containing an emulsifier, and add Dextran-70 to it to adjust the pH of the solution to 8;
[0036] 5. quickly drop the organic phase prepared in step 2 to the external aqueous phase prepared in 4;
[0037] ⑥ Removing the organic solvent to obtain a colloidal solution of nanoparticles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com