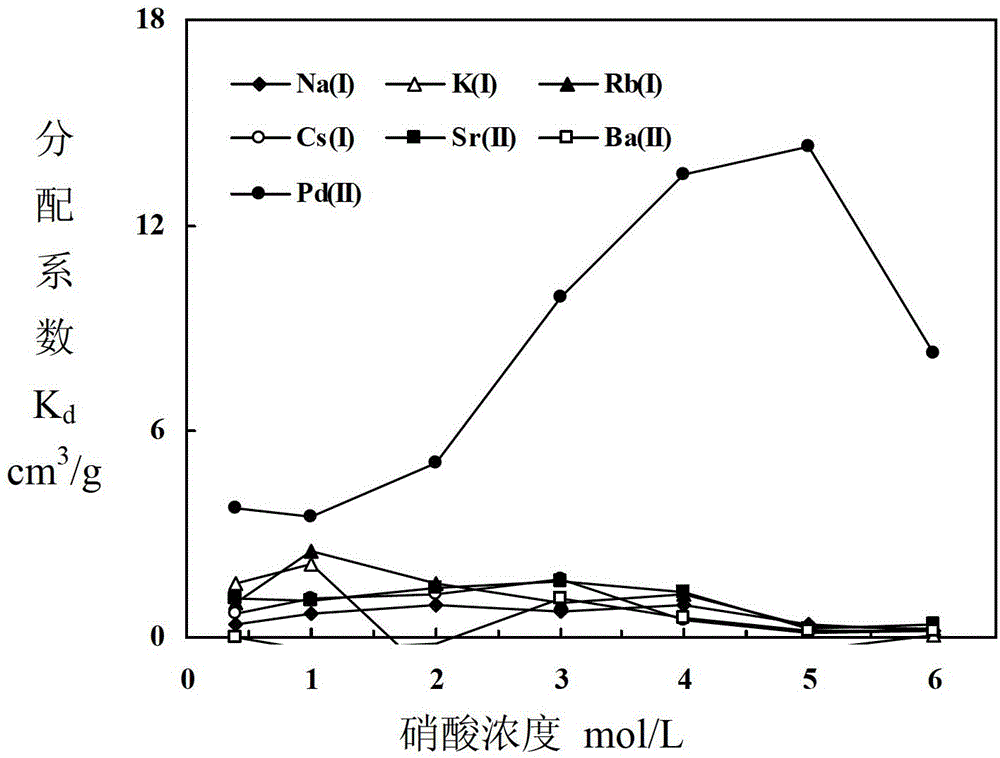
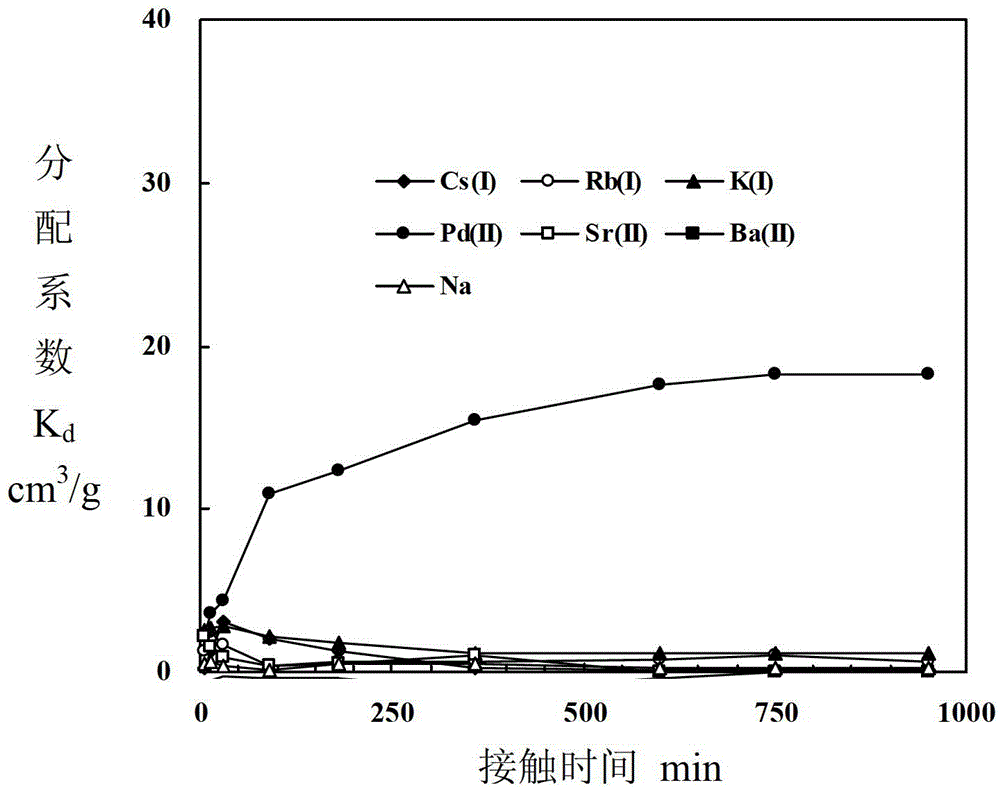
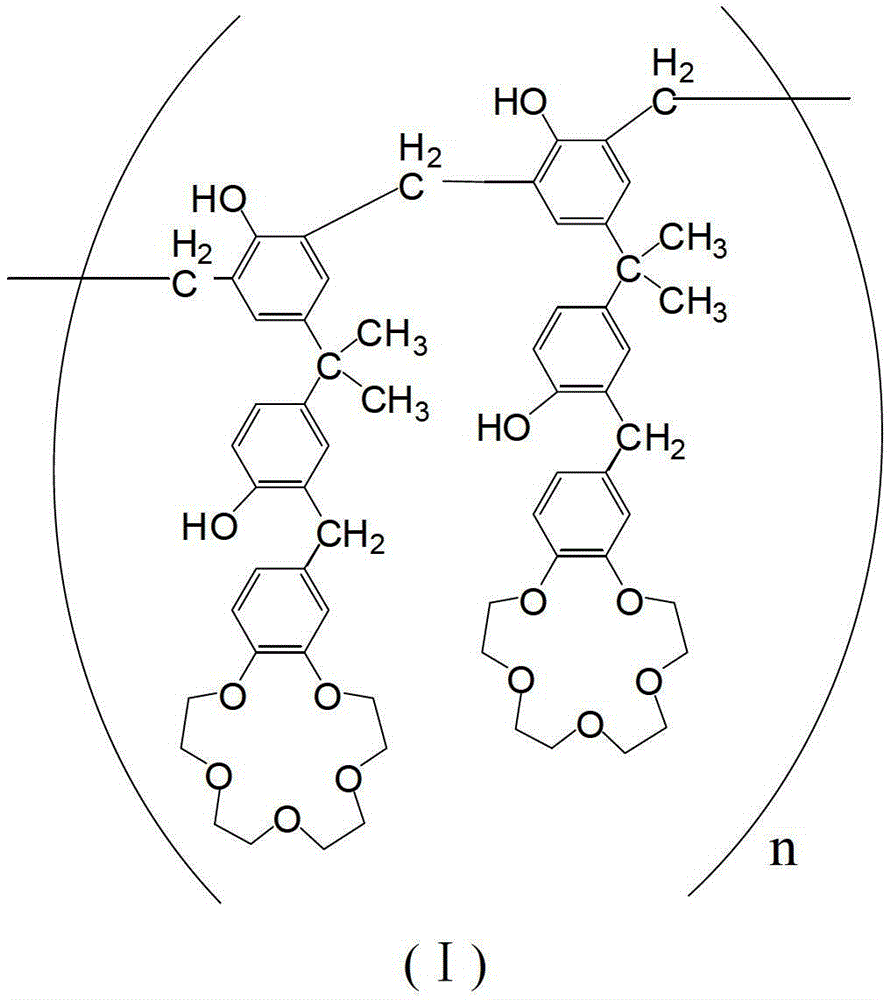
Adsorbent for separating palladium from alkali metals and alkaline-earth metals and its preparation method and use
A technology of alkaline earth metal and adsorbent, applied in the field of adsorbent for separating element palladium and its preparation, can solve the problems of high acidity, high radioactivity, high toxicity, etc., achieve fast separation speed, good selectivity, and reduce complexity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of 4-[3-(3-benzo-15-crown-5)methyl-4-hydroxy-phenyl]methylethyl-phenol resin
[0037] Add 3.42g (15mmol) 2,2-bis(4-hydroxyphenyl) propane and 3.49g (13mmol) benzo-15-crown 5 to 30g trichloroacetic acid, stir and dissolve at 90°C to form a solution, then Add 1.79g of paraformaldehyde (containing 60mmol formaldehyde) into the solution within 4.5 hours. After the addition is completed, the reaction temperature is raised to 140°C. After 4 hours of reaction, precipitation begins to appear in the reaction system, and the mass to be precipitated no longer increases. Cool the reaction system to room temperature, add 150mL of methanol to the resulting reaction solution to atomize the precipitate, crush and filter to obtain a solid powder, continue to wash the solid powder with 50mL of methanol, and repeat the washing several times until the cleaning solution (i.e. methanol ) without color change, finally obtained brown powder, which is 4-[3-(3-benzo-15-crown-5)methyl-4...
Embodiment 2
[0040] The preparation of embodiment 2 adsorbents
[0041] 100 grams of 4-[3-(3-benzo-15-crown-5)methyl-4-hydroxyl-phenyl]methylethyl-phenol resin (n is 7) with structural formula (I) was dissolved in In 1000mL of dichloromethane, mix evenly; add 150g of porous silica gel, stir evenly to volatilize most of the dichloromethane until the material is in a nearly dry state, and then dry the nearly dry material in vacuum at 45°C for 24h.
Embodiment 3
[0042] The preparation of embodiment 3 adsorbents
[0043] 100 grams of 4-[3-(3-benzo-15-crown-5)methyl-4-hydroxyl-phenyl]methylethyl-phenol resin (n is 9) with structural formula (I) was dissolved in In 1000mL of dichloromethane, mix well; add 200g of porous silica gel, stir evenly to volatilize most of the dichloromethane until the material is in a nearly dry state, and then dry the nearly dry material in vacuum at 45°C for 24h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com