Ru and / or Ir noble metal oxide and application thereof to oxygen evolution electro-catalysis
A precious metal oxide, precious metal salt technology, applied in metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, chemical/physical process, etc., can solve application limitations, cumbersome steps, complicated operation process, etc. problems, to achieve high preparation efficiency, extended application scope, economical and reasonable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh 4g of surfactant P123 and dissolve it in 93g of deionized water, add 25g of hydrochloric acid with a concentration of 37wt.%, stir at 40°C for 2h, add dropwise 8.8g of tetraethyl orthosilicate (TEOS), stir for 24h, and move to a high-temperature reaction kettle in 120°C for 24 hours, cooled naturally, filtered, the solid powder was washed with deionized water until no foam, and dried at 60°C for 24 hours to prepare silica molecular sieve SBA-15.
[0034] Disperse 2.5g of SBA-15 in 150mL of 1wt.% APS ethanol solution, stir at room temperature for 4h, filter, wash with absolute ethanol, repeat 5 times, and dry in vacuum at 60°C to obtain amino-modified silica molecular sieves SBA-15. Take 0.45g of amino-modified SBA-15 and disperse it in 40mL of deionized water, add H 2 IrCl 6 , so that the total noble metal ion concentration is 0.08M, after stirring and impregnating for 4h, at 50°C, N 2 Add dropwise 1 M NaBH under protection 4 solution to make NaBH 4 The molar...
Embodiment 2
[0036] Adopt the same method of embodiment 1 to test, and embodiment 1 difference is, but add the RuCl that contains 3~5 crystalline waters 3 with H 2 IrCl 6 Replace H 2 IrCl 6 , where the precursor molar ratio of Ru to Ir is 1:4, the preparation of Ru 0.2 Ir 0.8 o 2 , denoted as S-Ru 0.2 Ir 0.8 o 2 .
Embodiment 3
[0038] Adopt the same method of embodiment 1 to test, and embodiment 1 difference is, but add the RuCl that contains 3~5 crystalline waters 3 with H 2 IrCl 6 Replace H 2 IrCl 6 , where the precursor molar ratio of Ru to Ir is 4:1, the preparation of Ru 0.8 Ir 0.2 o 2 , denoted as S-Ru 0.8 Ir 0.2 o 2 .
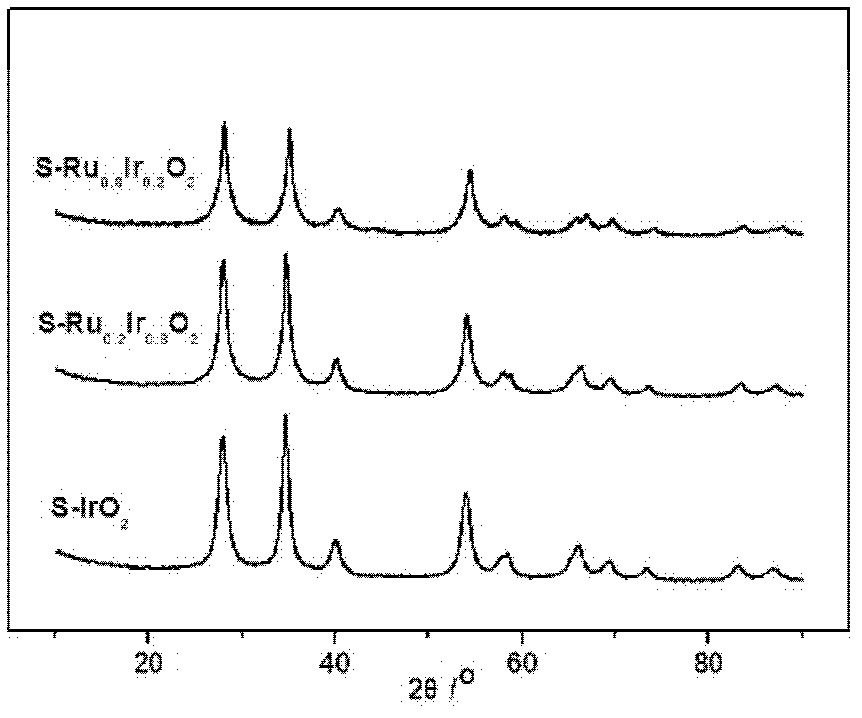
[0039] From figure 1 It can be seen that the S-IrO prepared in Example 1 2 It has a nanorod-like structure, indicating that it replicates the shell layer mechanism of SBA-15. From figure 2 It can be seen that the catalysts prepared in Examples 1 to 3 have a typical rutile phase structure, calculated by the Sherrer formula, S-Ru x Ir 1-x o 2 The average grain size is 5-7nm.
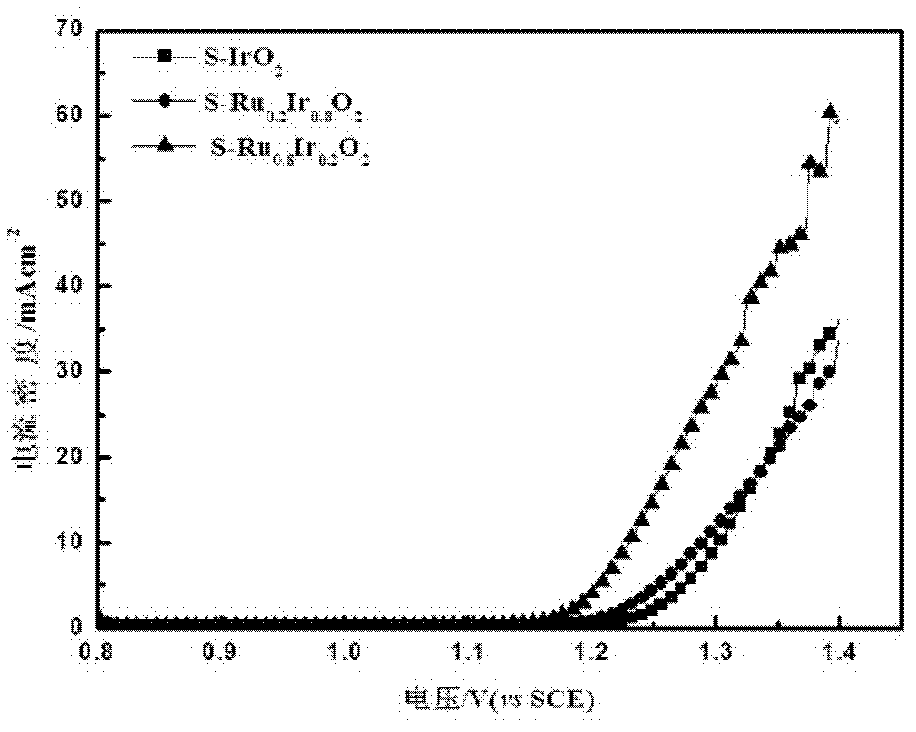
[0040] From image 3 It can be seen from the linear scan curve of S-Ru x Ir 1-x o 2 The oxygen evolution electrocatalytic activity was enhanced with the increase of Ru content, especially near the onset oxygen evolution potential.
[0041] The catalyst prepared in Examples 1-3 was used...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com